Research Article - (2024) Volume 19, Issue 4
Validity of synovial fluid lipid parameters and Apolipoprotein a1 as biochemical markers of advanced knee osteoarthritis among middle Euphrates Iraqi women
Ali Ameer Hamzah1*, Ali H. Gheni2 and Jawad K. Abbas3Received: 02-Aug-2024, Manuscript No. gpmp-24-145936; Editor assigned: 03-Aug-2024, Pre QC No. P-145936; Reviewed: 15-Aug-2024, QC No. Q-145936; Revised: 29-Aug-2024, Manuscript No. R-145936; Published: 30-Dec-2024
Abstract
Background: Several biomarkers are examined to predict the severity and advanced stages of knee osteoarthritis, however; yet, no biomarker is the optimal one. With some advantages and disadvantages. In Iraq, there is a lack in the studies that investigated lipid profile in the synovial fluid and its role in the assessment of Knee Osteoarthritis (KOA) severity or grade.
Objective: To assess the validity of lipid profile parameters and Apolipoprotein A1 as biochemical markers for progression of knee osteoarthritis among Middle Euphrates Iraqi Women. Patients and Methods: A cross-sectional study conducted in Al-Najaf Al-Ashraf Teaching Hospital and Al-Sader Teaching Hospital, in Al-Najaf City during a period 2021-2023, included 118 Women with KOA of Kellgren-Lawrence (KL) grades 2 to 4. Synovial fluid and blood samples were collected and sent for laboratory investigations. Data were analyzed using the statistical package for social sciences version 28 at a significance level of 0.05.
Results: The mean serum and synovial fluid Triglyceride (TG) levels were significantly higher, in Women with KL-grade 4 than those with KL grade 3 and grade 2. Serum and Synovial fluid HDL-C and APOA1 levels were significantly lower in Women with advanced KL grade, (P. value=0.001). Synovial fluid TG showed a sensitivity of 76.5%, specificity 73.2% and accuracy of 74.7%. The synovial fluid HDL-C showed a sensitivity, specificity and accuracy of 79.3%, 81.3% and 80.7%, respectively. Synovial fluid APO A1, had a sensitivity of 77.2%, specificity of 79.6% and accuracy of 79.7%.
Conclusion: Synovial fluid high density lipoprotein cholesterol, triglycerides and Apolipoprotein A1 were good biochemical markers of advance knee OA and can be promising predictor in clinical evaluation and follow up of diseases progression among Iraqi patients with knee OA.
Keywords
Osteoarthritis; Triglyceride; Apolipoprotein A1; Kellgren-Lawrence grades; Synovial fluid
Introduction
Osteoarthritis (OA) is the most common disease of the joints worldwide, it is accompanied by chronic pain, various degrees of functional limitation, a high level of disability, and a decrease in the quality of life from other point of view, the knee joint is the comments affected joint [1]. Globally, the pooled prevalence of KOA was 16% in 2020 among population older than 15 years, however, the prevalence increases with age to reach almost 23% in individuals aged 40 years or older. From other point of view, the pooled global incidence rate of KOA was 203/10000 person-year and the total cases reported worldwide were 654 million in 2020, with varied rates of incidence and prevalence among countries [2]. In the Middle East, the number of reported cases was almost 18 million in 2019 which is almost triple that reported earlier in 1990. Moreover, KOA about 1.7 million cases incident in 2019 alone in this region [1].
To date, there is a significant amount of evidence regarding the direct connection of knee osteoarthritis with hereditary predisposition, obesity, aging, mechanical stress and inflammation, however, the entire chain of links of pathogenesis and the reasons for the significant prevalence of OA are not definitively established. The lack of clear ideas about the factors that initiate the onset or exacerbation of the disease hinders efforts to prevent and effectively treat KOA [2,3]
The main factors contributing to the fairly rapid spread of KOA are the increase in life expectancy and the "epidemic" of obesity. The aging of the population is accompanied by an increase in cumulative tissue damage of compromised joints in the context of progressive changes in the musculoskeletal system, which leads to an increase in degeneration of the knee joint with age Individuals with a high body mass index (BMI) have the combined effects of lower extremity joint overload and dyslipidemia-induced inflammation [4-7].
As the fact that KOA is an incurable progressive disease, in late, advanced and severe and end stage disease, patients may need total knee arthroplasty. This high-tech operation requires significant economic costs and medical and social adaptation and has a certain number of postoperative complications and adverse outcomes [8], therefore, rheumatologists, orthopedic surgeon and even general practitioners are continuously aware about the prevention, diagnosis and proper management of KOA at early stages of the disease as it is recommended by several guidelines [9,10]. In clinical practice, hence, preventive and management strategies based mainly on the modifiable risk factors that contribute to KOA and looking for optimal markers and predictors of severe disease stage in order to offer efficient treatment measures. Therefore, different biomarkers are investigated by many authors aiming to find a prognostic biomarker that can be used clinically and to recognize the progression of disease at different stages. The main goal of these investigations is to clarify and well understanding the pathogenesis of the disease and to find an ultimate development of disease-modifying OA drug [11]. Results of these studies are not yet definitely conclusive and none of the tested biomarker can be considered as the ideal or the gold standard to predict disease progression and severity. Therefore, further searching for the best biomarker or predictor is taken into account by the scientific community of rheumatologist. In the last 5 years, some studies have shown promising markers based on the patient’s data and X-ray imaging for monitoring of patients. Different biomarkers were examined like synovial fluid adipokines, white blood cells, inflammatory markers, other studies compared the synovial fluid markers vs. serum levels of these markers [12-15].
Testing for the High density Lipoprotein (HDL) cholesterol and Apolipoprotein A1 in the synovial fluid have been documented to play a significant role in assessment of severity of KOA and can be considered as potential predictors of severe disease form in patients with primary KOA [16]. In Iraq, studies concerned with this topic are lacking and we did not found relevant studies, hence we believed that this is the first study in Iraq. Our aim is to investigate the levels of HDL-c and Apolipoprotein A in synovial fluid and to evaluate their association with severity and progression of disease in comparison to clinical and radiological parameters to get evidence about their utility in clinical practice as potential predictors for diseases progression and severity.
Patients and Methods
This was a cross-sectional study conducted during a period of 3 years, from January 2021 to December 2023 at the departments of Rheumatology and Rehabilitation in two main Teaching hospitals; Al-Najaf Al-Ashraf and Al-Sader Teaching Hospitals in Al-Najaf Al-Ashraf city, Middle of Iraq.
Patients: A total of 118 women of both genders were enrolled in this study who had primary knee osteoarthritis and consented to perform the procedure and participate in the study.
Inclusion criteria: Iraqi adult patients presented with proved diagnosed knee OA of different duration and met the clinical and radiological criteria of the American college of rheumatologists. Both genders were included
Exclusion criteria: Patient was excluded from the study if he/she had one or more of the following:
- Kellgren-Lawrence (KL) grade of 0 and 1.
- Refused SF aspiration from their knee joints
- Rheumatoid arthritis
- Secondary osteoarthritis of the knee joint
- Traumatic injury or surgical intervention on the knee joint
- Chromic knee joint pain of unknown etiology
- Autoimmune diseases or systemic inflammatory disorders
- Malignant diseases
- Chronic kidney disease at any stage
- Acute or chronic liver diseases
- Received lipid lowering agents; and
- Immunocompromised, or receiving immunosuppressant agents, cytotoxic or biological treatment.
Study protocol and ethical issues
- The official agreement was obtained from the scientific committee and the Research Ethics committee in Al-Najaf Health Directorate, Department of Training and Development.
- Signed informed consent obtained from each participant before he/she was included in the study and prior to perform any intervention.
- Venous blood samples were collected under aseptic condition by trained laboratory staff and transferred to the laboratory for processing and analysis under high quality measures.
- Synovial fluid (SF) samples were collected through direct aspiration of SF using a syringe and an intraarticular needle was inserted and introduced within the knee joint between the joint space, when the place of the needle is assured, then the synovial fluid was aspirated. When the direct aspiration failed, small volume lavage with 10 ml saline was performed. However, we took into account the dilution of SF due to saline lavage and the laboratory staffs was informed for correction of concentrations using correction lab methods like urea based method [17].
Clinical assessment:
Knee OA was proved diagnosed according to the standard criteria, based on clinical examination, radiological and laboratory findings.
Severity of disease was assessed based on radiography and symptomatic assessment tools; radiographically, we performed a standing anteroposterior x-ray for all patients and the disease’s severity was graded according to the Kellgren-Lawrence (KL) grading system [18,19]. For the purpose of our study, patients with less than KL grade 2 were assigned as a reference group.
Pain intensity was assessed using the Visual Analogue Scale (VAS) and the patient was asked to rate his/her knee pain on a scale of 10 cm where 0 represents none and the 10 point represents the extreme pain that can be imagine. Pain intensity was considered mild when the VAS score of 3.4 or less, moderate (VAS score of 3.5 to 7.4) and severe when the VAS score more than 7.4 [20,21].
Laboratory tests and analysis: Serum lipid profile was investigated including the serum triglycerides level, total serum cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), Apolipoprotein A1. Similarly, quantitative analysis of these parameters in synovial fluid was performed.
Statistical analysis: Data were entered, managed and analyzed using the Statistical Package for Social Sciences (SPSS) software for windows version 28. All scale, quantitative variables were tested for statistical normal distribution using one-sample Kolmogorov-Smirnov test. Parametric student’s t test for two independent samples used to compare means between two subgroups. Analysis of variances (ANOVA) test used to compare means of a scale variable among three or more subgroups. Nonparametric tests, Mann-Whitney and Kruskal Wallis Test used as an alternative when the scale variables did not follow the normal distribution. Chi-square test and alternatively, Fisher’s exact test used to compare nominal or ordinal variables. Bivariate Pearson’s and Spearman’s tests used in correlation analysis accordingly. Receiver Operating Characteristics (ROC) curve analysis used to assess the validity parameters of the markers in prediction of advanced KOA. All statistical tests and procedures performed at a two tailed level of significance of ≤ 0.05.
Results
A total of 118 women were enrolled in this study with a mean age of 51.8 ± 9.2 years and 59.3% of them were older than 50 years. Overweight and obese patients contributed for 44.1% and 32.2%, respectively. Both knees affected in 57.6%. Duration of disease ranged from one year to more than 6 years with a mean of 3.7 ± 2.5 years, these findings are shown in (Tab. 1.).
| Variable | No. | % | |
|---|---|---|---|
| Age (year) | ≤ 40 | 14 | 11.9 |
| 41-50 | 34 | 28.8 | |
| 51-60 | 41 | 34.7 | |
| >60 | 29 | 24.6 | |
| Mean (SD) | 51.8 (9.2) | - | |
| BMI | Normal | 28 | 23.7 |
| Overweight | 52 | 44.1 | |
| Obese | 38 | 32.2 | |
| Mean (SD) (kg/m²) | 27.6 (4.6) | - | |
| Laterality | Left | 22 | 18.6 |
| Right | 28 | 23.7 | |
| Bilateral | 68 | 57.6 | |
| Duration of KOA (year) | 1-2 | 32 | 27.1 |
| 3-4 | 47 | 39.8 | |
| 5- 6 | 26 | 22.0 | |
| >6 | 13 | 11.0 | |
| Mean (SD) | 3.7 (2.5) | - | |
| SD: Standard Deviation | |||
Tab. 1. Baseline characteristics of the studied group (N=118).
Among the studied group, 32.2% had KL-grade 2, 41.5% KL-grade 3 and 26.3% had KL-grade 4. According to the VAS scores, moderate pain reported by 64.4% of the patients and severe pain scores reported by 35.6% while none of the patients showed mild pain scores, however, the mean VAS score was 6.3 ± 1.4, (Tab. 2.).
| Variables | No. | % | |
|---|---|---|---|
| KL grade | Grade 2 | 38 | 32.2 |
| Grade 3 | 49 | 41.5 | |
| Grade 4 | 31 | 26.3 | |
| VAS score* | Moderate | 76 | 64.4 |
| Severe | 42 | 35.6 | |
| Mean (SD) | 6.3 (1.4) | - | |
*: None of the patients had mild
Tab. 2. Distribution of the studied group according to the Kellgren-Lawrence and VAS scores.
Tab. 3. demonstrates the mean values of lipid parameters in the serum and synovial fluid.
| Variables | Serum | SF | ||
|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD |
| Triglycerides (mg/dL) | 157.8 | 27.6 | 27.7 | 6.6 |
| Total Cholesterol (mg/dL) | 204.9 | 37.4 | 57.4 | 10.5 |
| HDL-C (mg/dL) | 42.3 | 11.7 | 16.2 | 5.1 |
| LDL-C (mg/dL) | 150.9 | 27.7 | 36.2 | 6.7 |
| Apo A1 (mg/dL) | 162.1 | 32.8 | 47.2 | 13.6 |
Tab. 3. Lipid profile and APO A1 in the serum and synovial fluid of 118 KOA patients.
Comparison of lipid parameters and APO A1 in the serum and synovial fluid according to KL-grade revealed that serum triglycerides was significantly increased with the advancing KL-grade; higher mean serum TG of 171.4 ± 22.0 mg/dL reported in patients with KL-grade 4 compared to 157.6 ± 23 mg/dL in those with KL-grade 3and the lower mean TG of 146.8 ± 32.5 mg/dL reported in those with KL grade 2, (P. value=0.001, significant). Similar trend found in synovial fluid TG; the mean SF. TG was 30.9 ± 5.3, 27.6 ± 5.5 and 25 ± 7.8 mg/dL, respectively, (P. value=0.001, significant). Both serum and Synovial fluid HDL-C levels were significantly lower in patients with higher KL grade, in both comparisons, (P. value=0.001, significant). So as for the synovial fluid APO A1 levels (P. value=0.001, significant). Other parameters including serum and synovial fluid total cholesterol, LDL-C and serum APO A1 were not significantly different across KL grading, (P>0.05, not significant), (Tab. 4.) and further graphically presented in (Fig. 1.2.3.4. & 5.).
| Variables | kl Grade 2 | kl Grade 3 | kl Grade 4 | P value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| S. Triglycerides (mg/dL) | 146.8 | 32.5 | 157.6 | 23.0 | 171.4 | 22.0 | 0.001 |
| S. Total Cholesterol (mg/dL) | 201.5 | 43.2 | 205.2 | 31.9 | 208.5 | 38.8 | 0.749 |
| S. HDL-C (mg/dL) | 47.4 | 10.7 | 41.4 | 10.1 | 37.5 | 13.1 | 0.001 |
| S. LDL-C (mg/dL) | 147.2 | 21.9 | 151.5 | 31.8 | 154.3 | 27.6 | 0.561 |
| S. Apo A1 (mg/dL) | 166.0 | 34.6 | 161.3 | 30.1 | 158.6 | 35.3 | 0.639 |
| SF. Triglycerides (mg/dL) | 25.0 | 7.8 | 27.6 | 5.5 | 30.9 | 5.3 | 0.001 |
| SF. Total Cholesterol (mg/dL) | 56.4 | 12.1 | 57.4 | 8.9 | 58.4 | 10.9 | 0.749 |
| SF. HDL-C (mg/dL) | 20.6 | 3.8 | 15.7 | 3.4 | 11.4 | 4.2 | 0.001 |
| SF. LDL-C (mg/dL) | 35.3 | 5.2 | 36.4 | 7.6 | 37.0 | 6.6 | 0.561 |
| SF. Apo A1 (mg/dL) | 54.1 | 17.5 | 47.4 | 9.8 | 38.6 | 6.8 | 0.001 |
Tab. 4. Comparison of lipid parameters and APO A1 in the serum and synovial fluid according to Kellgren-Lawrence grade (N=118).
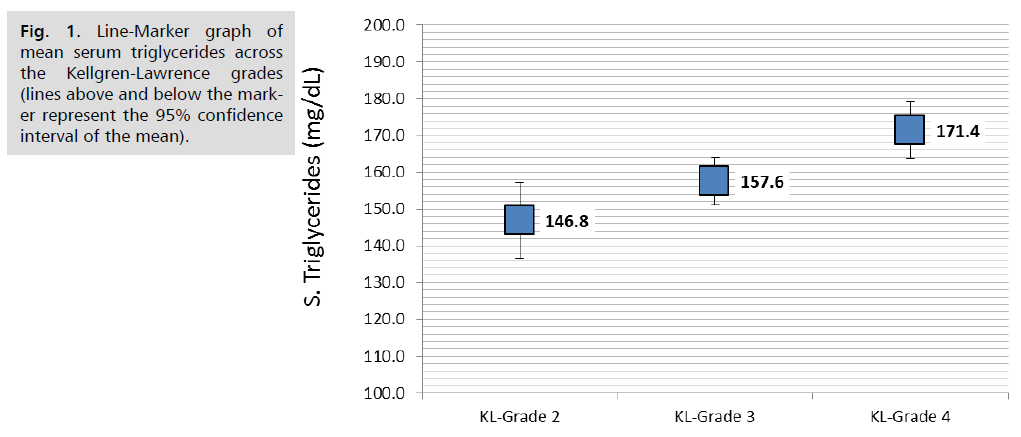
Fig. 1. Line-Marker graph of mean serum triglycerides across the Kellgren-Lawrence grades (lines above and below the marker represent the 95% confidence interval of the mean).
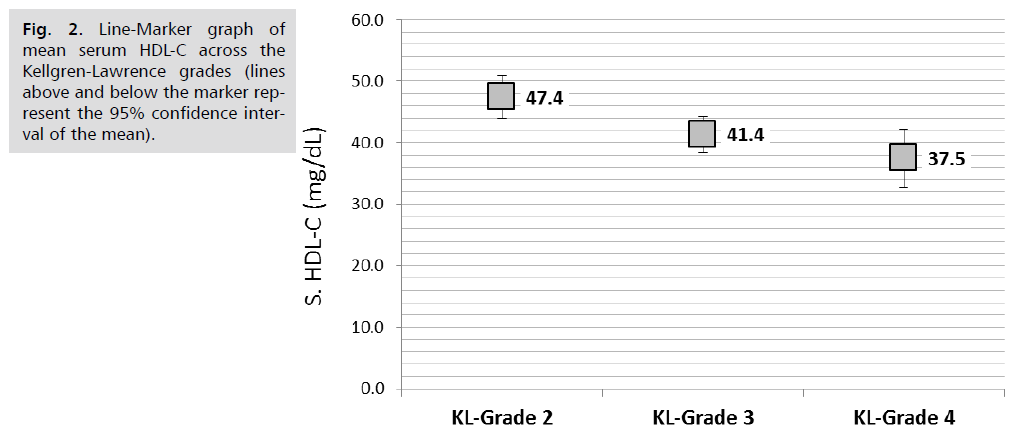
Fig. 2. Line-Marker graph of mean serum HDL-C across the Kellgren-Lawrence grades (lines above and below the marker represent the 95% confidence interval of the mean).
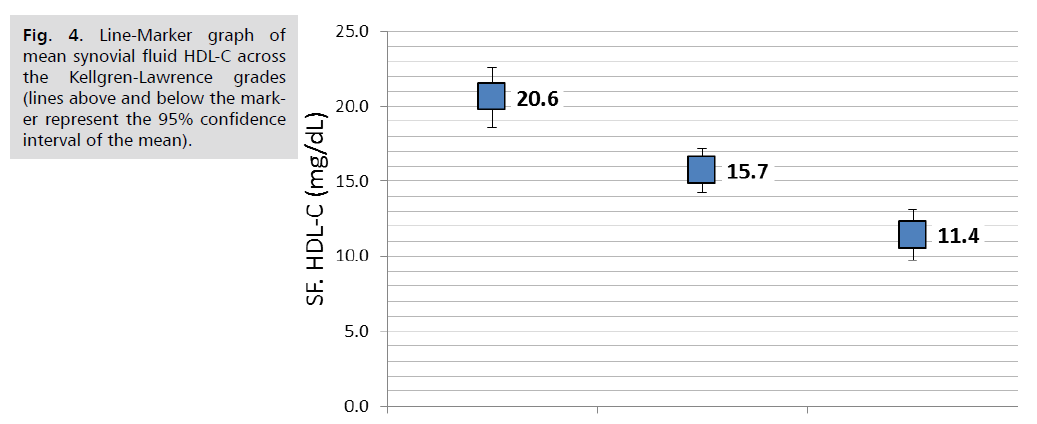
Fig. 3. Line-Marker graph of mean synovial fluid triglycerides across the Kellgren-Lawrence grades (lines above and below the marker represent the 95% confidence interval of the mean).
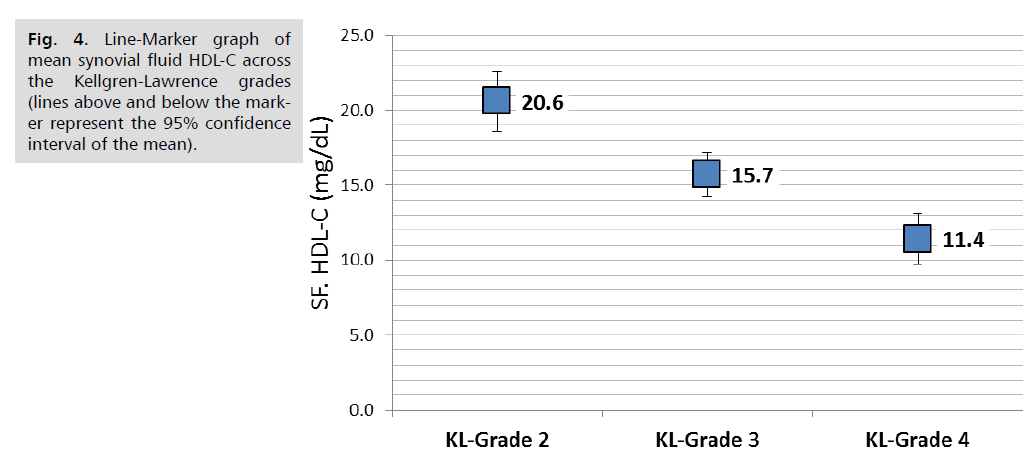
Fig. 4. Line-Marker graph of mean synovial fluid HDL-C across the Kellgren-Lawrence grades (lines above and below the marker represent the 95% confidence interval of the mean).
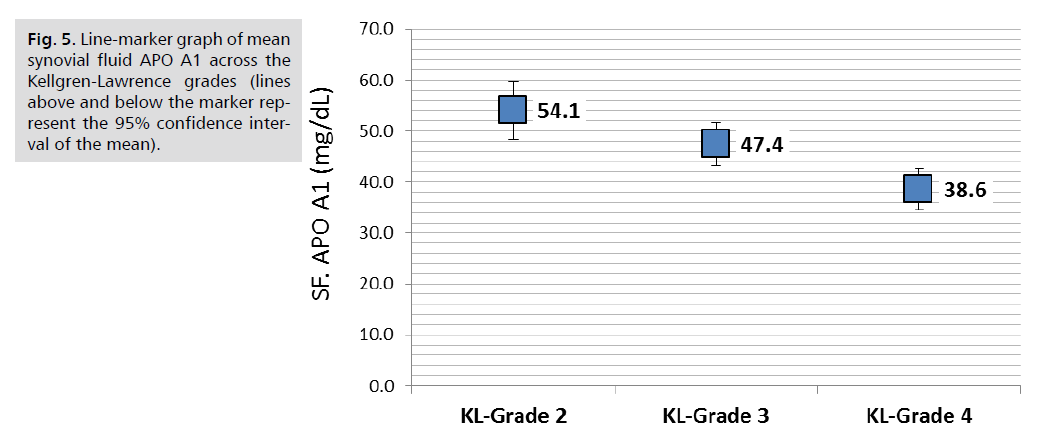
Fig. 5. Line-marker graph of mean synovial fluid APO A1 across the Kellgren-Lawrence grades (lines above and below the marker represent the 95% confidence interval of the mean).
Receiver operating characteristics (ROC) curve analysis showed that synovial fluid TG, HDL-C and APO A1 was good predictor of advanced KOA with an area under the ROC curve of 0.762, 0.879 and 0.806, respectively. Synovial fluid TG showed a sensitivity of 76.5%, specificity 73.2% and accuracy of 74.7%. Synovial fluid HDL-C showed higher sensitivity, specificity and accuracy of 79.3%, 81.3% and accuracy of 80.7%, respectively. SF. APO A1 showed a sensitivity of 77.2%, specificity of 79.6% and accuracy of 79.7%. Nonetheless, the comparison of these validity parameters did not show significant difference between the three parameters, (P>0.05, not significant) as shown in (Fig. 6. and Tab. 5.).
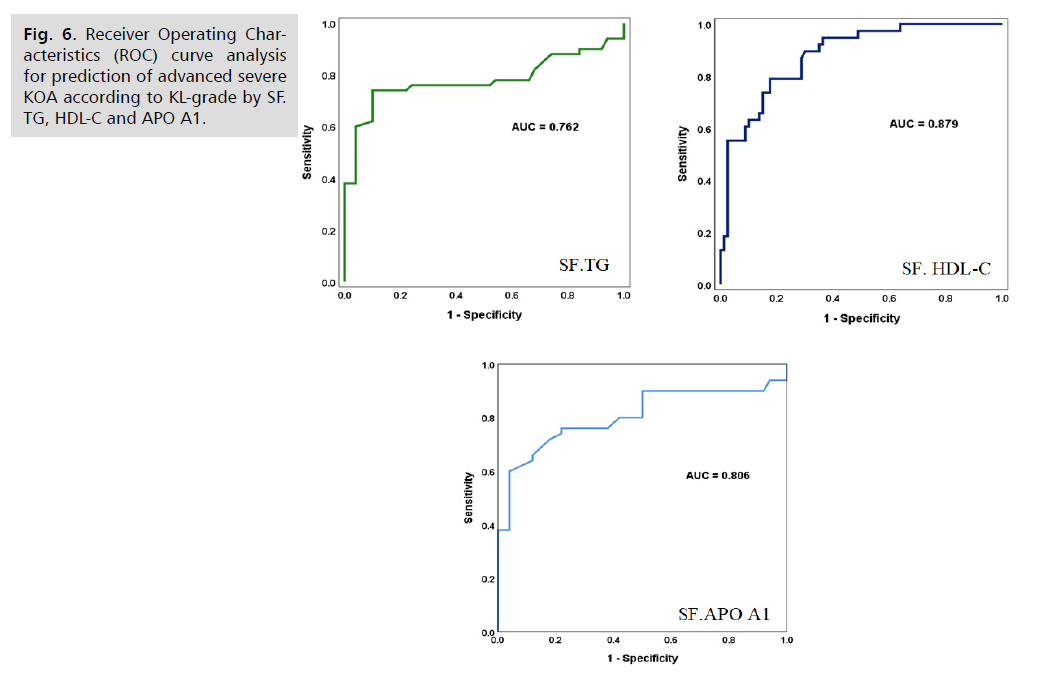
Fig. 6. Receiver Operating Characteristics (ROC) curve analysis for prediction of advanced severe KOA according to KL-grade by SF. TG, HDL-C and APO A1.
| Variables | Sensitivity | Specificity | Accuracy |
|---|---|---|---|
| SF. TG | 76.50% | 73.20% | 74.7% |
| SF. HDL-C | 79.30% | 81.30% | 80.7% |
| SF. Apo A1 | 77.20% | 79.60% | 79.7% |
| P. value | 0.865 | 0.832 | 0.992 |
Tab. 5. Sensitivity, specificity and accuracy of SF. TG, HDL-C and APO A1 as predictor of advanced KL-grade of KOA.
Bivariate correlation analysis showed no significant association between age and each of VAS score, SF. TG, HDL-C or APO A1, (P. value >0.05). Female gender was significantly associated with higher SF. HDL-C (R=0.202, P. value=0.028) but not with other parameter or VAS score, (P>0.05).
Higher BMI significantly correlated with higher VAS scores (R=0.372, P. value=0.002), lower HDL-C (R=-0.246, P. value=0.007) and lower APO A1 (R=-0.206, P. value=0.025). However, according to the R values, all of these correlations were weak (<0.4) despite they were significant. Additionally, we found that higher VAS score was significantly correlated with lower HDL-C levels (moderate correlation; R=-0.425, P. value=0.001), also VAS score was significantly but weakly correlated with SF. APO A1 level (R=-0.352, P. value=0.001), (Tab. 6.).
| Variables | Correlation indicator | VAS score | SF. TG | SF. HDL-C | SF. Apo A1 |
|---|---|---|---|---|---|
| Age (older) | R | -0.081 | -0.073 | -0.109 | -0.082 |
| P. value | 0.384 | 0.433 | 0.240 | 0.379 | |
| BMI (higher) | R | 0.372 | 0.074 | -0.246 | -0.206 |
| P. value | 0.002 | 0.427 | 0.007 | 0.025 | |
| Disease duration | R | 0.040 | -0.048 | -0.069 | -0.017 |
| P. value | 0.668 | 0.606 | 0.457 | 0.856 | |
| VAS score | R | - | 0.118 | -0.425 | -0.352 |
| P. value | - | 0.203 | 0.001 | 0.001 |
Tab. 6. Results of bivariate correlation analysis for the correlation between patients characteristics and predictors of advanced KOA.
Discussion
Several biomarkers are investigated to predict the severity and advanced stages of knee OA, nonetheless, none of these biomarkers is the optimal or ideal with some advantages and disadvantages [12-15], therefore, scientific community still looking for new and promising biomarkers that help in follow up and management of patients with KOA. In Iraq there is lacking in studies that investigated biomarkers of knee OA and to best of our knowledge, this is the first study in the field of rheumatology that examine the role of lipid parameters and APO as biomarkers that can predict the progression of disease in patients with KOA, these factors encouraged conducting our current study.
In the current study we included 118 Iraqi patients with different stages of KOA according to Kellgren-Lawrence grading system. In more than half of our patients both knees were affected.
The baseline characteristics of the studied group revealed that almost 60% of them aged older than 50 years and 68.6% were females, these findings were not unexpected and consistent with the clinical and epidemiological pictures of knee OA where the prevalence of KOA increased with advancing age and among more frequent in women than men [22,23]. Our findings consistent with demographic characteristics of KOA patients reported in previous Iraqi studies [24,25]. From other point of view, the age standardized prevalence of OA in Iraq is considered among the lower rates compared to other countries in the region [26].
In our study we excluded patients with no radiological findings of OA (KL-grade 0) and those who had doubtful narrowing (KL-grade 1) and therefore all our patients with KL-grade 2, 3 or 4. With regard to the VAS score we use it as the simplest method to evaluate pain in these patients and also because some authors suggested that VAS scoring is more sensitive than WOMAC in detecting pain differences [22]. We found that the mean VAS score is relatively high, 6.3 ± 1.4, and none of the patients has mild or no pain where almost two thirds of the patients had moderate pain with a VAS score between 3.5 and 7.4 and the remaining patients had severe pain with a VAS score of 7.5 or higher. Almost similar mean VAS score reported by Parkes, et al. [27] who found that the mean VAS score for pain on nominated activity was 6.6 ± 1.8 and it was the more sensitive to change of patients-preference measures for pain.
When we compared the lipid parameters and APO A1 in the serum and synovial fluid according to Kellgren-Lawrence grade we found that serum and SF TG levels were both significantly high in advanced KL grades In contrast, both of serum and SF HDL-C levels were significantly lower in patients with higher KL grades. On the other hand, SF. APO A1 was significantly reduced with advancing KL-grade, while serum APO A1 was not significantly different across the KL-grades. Furthermore, we did not find a significant difference in the mean values of serum or SF. Total cholesterol or LDL-C across the KL-grades. Further analysis was performed using the ROC curve analysis and showed that synovial fluid TG, HDL-C and APO A1 were good predictor of advanced and severe KOA and they produced good sensitivity, specificity and accuracy rates as markers of advanced KOA grade. We demonstrated that these parameters are independently associated with radiographic severity and symptoms of KOA among our cohort. However, no such association was found with serum levels, hence, synovial fluid TG, HDL-C and APO A1 showed an exceptional efficacy in detection of advanced phase of KOA. These findings may reflect an effect for this parameter in the development and pathogenesis of KOA and they can act as novel biochemical indicators for assessment of severity of KOA. The importance of HDL came from the fact that it is an essential element in the lipoprotein transport system and plays an important role in the controlling the metabolism of lipid and hemostasis in addition to its inhibitory activity on the cytokines and it’s an anti-inflammatory effect [28]. Additionally, many previous studies suggested an association between development of OA and dyslipidemia and attributed their finding to the anti-inflammatory effect of high levels of HDL and the pro-inflammatory effect of LDL-C and its oxidized form [29]. In an earlier clinical study, Oliviero, et al. demonstrated a substantial reduction in the serum level of ApoA1 in OA patients [30]. According to gene expression studies, there is a downregulation in the cholesterol influx gene in the chondrocytes of OA patients; this gene is responsible for the APOA1 encoding.
The fact that the joint is bathed in synovial fluid leads to a strong evidence that changes in the composition of synovial fluid can be significantly associated with joint diseases, this offers a chance to the synovial fluid parameters to be promising markers of the joint pathologies [15]. Our study revealed that synovial fluid HDL-C was superior to other two parameters followed by APO A1 and the least predictor was TG. However, for the three parameters the sensitivity ranged between 76.5%-79.3%, specificity, 73.2%-81.3% and accuracy ranged between 74.7%-80.7%. These validity rates considered good rates and that the three parameters can be promising biochemical markers that are simply investigated in the hospital laboratory with no complex procedures and low cost. In our study, neither serum nor synovial total cholesterol and LDL-C showed significant correlation with the radiographic severity of KOA, which is supported by the findings of previous studies [29,30]
We further assessed association between characteristics of the patients on the SF. TG, HDL-C and APO A1 and VAS scores, we found no significant association between age and each of VAS score, SF. TG, HDL-C or APO A1. In contrast, we found a significant association between female and higher SF. HDL-C but not with other parameter or VAS score. Other findings is higher BMI significantly correlated with higher VAS scores, lower HDL-C level and lower APO A1, but all these correlations were weak as the R value was less than 0.4 Additionally, we found that higher VAS score was negatively correlated with SF. HDL-C levels and SF. APO A1 level. Previous studies documented that higher pain scores were significantly associated with advanced radiographic grade (KL grade) and SF biomarkers [12]
Furthermore, Schwager, et al. [29] did not found any significant association between HDL and radiographic or symptomatic OA.
Though the interesting findings, our study unavoidably encountered some limitations; initially, this investigation was carried out exclusively in only two hospitals and the number of participants was comparatively limited due to refusal of many patients to perform synovial fluid aspiration due to some misconception and erroneous believes about the procedure. Regrettably, we could not include a control group because we could not perform synovial fluid aspiration for healthy subjects due to ethical considerations. Furthermore, the cross-sectional study design prevented us from making conclusive causal inferences because we cannot approve the temporal relationship between OA and lipid profile of the patients. However, it is necessary to conduct extensive multicenter studies with larger sample size and longer duration taking into account the aforementioned limitations
Conclusion
Synovial fluid high density lipoprotein cholesterol, triglycerides and Apolipoprotein A1 were good biochemical markers of advance knee OA and can be promising predictor in clinical evaluation and follow up of diseases progression among Iraqi patients with knee OA. Hence we recommended the analysis of lipid profile in synovial fluid aspirates when performed for different reasons as a tool for follow up of disease progression.
Authors' Contribution
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- Hoveidaei AH, Nakhostin-Ansari A, Chalian M, et al. Burden of knee osteoarthritis in the Middle East and North Africa (MENA): an epidemiological analysis from 1990 to 2019. Arch Orthop Trauma Surg. 2023;143(10):6323-6333.
- Cui A, Li H, Wang D, et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29.
- Szilagyi IA, Waarsing JH, Schiphof D, et al. Towards sex-specific osteoarthritis risk models: evaluation of risk factors for knee osteoarthritis in males and females. Rheumatol. 2022 ;61(2):648-657.
- Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412-420.
- Runhaar J, Zhang Y. Can we prevent OA? Epidemiology and public health insights and implications. Rheumatol. 2018;57(suppl_4):iv3-9.
- Li D, Li S, Chen Q, et al. The prevalence of symptomatic knee osteoarthritis in relation to age, sex, area, region, and body mass index in China: a systematic review and meta-analysis. Front Med. 2020;7:304.
- Wluka AE, Lombard CB, Cicuttini FM. Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol. 2013;9(4):225-235.
- Madry H. Surgical therapy in osteoarthritis. Osteoarthr Cartil. 2022;30(8):1019-1034.
- Migliore A, Gigliucci G, Alekseeva L, et al. Treat-to-target strategy for knee osteoarthritis. International technical expert panel consensus and good clinical practice statements. Ther Adv Musculoskelet Dis. 2019;11:1759720X19893800.
- Wallis JA, Barton CJ, Brusco NK, et al. Exploring views of orthopaedic surgeons, rheumatologists and general practitioners about osteoarthritis management. Musculoskeletal Care. 2021;19(4):524-532.
- Attur M, Krasnokutsky-Samuels S, et al. Prognostic biomarkers in osteoarthritis. Curr Opin Rheumatol. 2013;25(1):136-144.
- Haraden CA, Huebner JL, Hsueh MF, et al. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res Ther. 2019;21:1-9.
- Calvet J, Orellana C, Gratacós J, et al. Synovial fluid adipokines are associated with clinical severity in knee osteoarthritis: a cross-sectional study in female patients with joint effusion. Arthritis Res Ther. 2016;18:1-1.
- Orellana C, Calvet J, Navarro N, et al. Higher synovial fluid white blood cell count in patients with knee osteoarthritis and metabolic syndrome. Osteoarthr Cartil. 2016;24:S84-5.
- Boffa A, Merli G, Andriolo L, et al. Synovial fluid biomarkers in knee osteoarthritis: a systematic review and quantitative evaluation using BIPEDs criteria. Cartilage. 2021;13(1_suppl):82S-103S.
- Zhang K, Ji Y, Dai H, et al. High-density lipoprotein cholesterol and apolipoprotein A1 in synovial fluid: potential predictors of disease severity of primary knee osteoarthritis. Cartilage. 2021;13(1_suppl):1465S-73S.
- Kraus VB, Stabler TV, Kong SY, et al. Measurement of synovial fluid volume using urea. Osteoarthr Cartil. 2007 Oct 1;15(10):1217-1220.
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
- Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474:1886-1893.
- Samma L, Rasjad C, Seweng A, et al. Correlation between Body Mass Index (BMI), Visual Analogue Scale (VAS) score and knee osteoarthritis grading. Med Clin Pract. 2021;4:100228.
- Boonstra AM, Preuper HR, Balk GA, et al. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain. 2014;155(12):2545-2450.
- da Costa BR, Saadat P, Basciani RM, et al. Visual Analogue Scale has higher assay sensitivity than WOMAC pain in detecting between-group differences in treatment effects: a meta-epidemiological study. Osteoarthr Cartil. 2021;29(3):304-312.
- Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152.
- Hussien H, Abdul Raheem Y. Risk factors and awareness about knee osteoarthritis among secondary school teachers. Med Sci. 2020;24(106):4031-4039.
- Jasim NA. Association of radiological osteoarthritis of the knee joint with locomotor disability. AL-Kindy Coll Med J. 2019;15(1):36-42.
- Shamekh A, Alizadeh M, Nejadghaderi SA, et al. The burden of osteoarthritis in the Middle East and North Africa region from 1990 to 2019. Front Med. 2022;9:881391.
- Parkes MJ, Callaghan MJ, O'Neill TW, et al. Sensitivity to change of patient‐preference measures for pain in patients with knee osteoarthritis: data from two trials. Arthritis Care Res. 2016;68(9):1224-31.
- Rye KA, Barter PJ. Regulation of high-density lipoprotein metabolism. Circ Res. 2014;114(1):143-56.
- Schwager JL, Nevitt MC, Torner J, et al. Association of serum low‐density lipoprotein, high‐density lipoprotein, and total cholesterol with development of knee osteoarthritis. Arthritis Care Res. 2022;74(2):274-280.
- Oliviero F, Sfriso P, Baldo G, et al. Apolipoprotein AI and cholesterol in synovial fluid of patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Clin Exp Rheumatol. 2009;27(1):79.
Author Info
Ali Ameer Hamzah1*, Ali H. Gheni2 and Jawad K. Abbas32Department of Medicine, Al-Najaf Teaching Hospital, Najaf, Iraq
3Department of Medicine, Kufa Medical School, Najaf, Iraq
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.