Research - (2022) Volume 0, Issue 0
Ulipristal acetate therapy vs uterine artery embolization in the management of uterine fibroids
Paul Naseef*, Ayman Abou Elnour, Sherif El Mekkawi and Salma NassarReceived: 16-Dec-2021, Manuscript No. gpmp-21-50006; Editor assigned: 17-Dec-2021, Pre QC No. P-50006; Reviewed: 26-Jan-2022, QC No. Q-50006; Revised: 06-Jun-2022, Manuscript No. R-50006; Published: 29-Sep-2022
Abstract
Aim: The aim of this study is to prove that UA is an effective line of management for uterine fibroids by causing a significant decline in fibroid volumes resulting in a substantial relief of fibroid-related symptoms, and to compare its results with those of uterine artery embolization.
Methods: This study is a randomized control trial that was conducted at Ain Shams University Maternity Hospital from March 2019 to March 2021 after approval of the research ethics committee (REC) of the Obstetrics and Gynecology Department, Ain Shams University. It included 70 women with symptomatic uterine fibroids who were randomly assigned into either Ulipristal Acetate (UA) group or uterine artery embolization (UAE) group (35 in each group). Both groups were followed up at 3 and 6 months after starting either treatment options to detect the decline in fibroid size as well as the improvement of symptoms.
Results: UA showed a statistically significant decline in dominant fibroid volume and diameter comparable to UAE at 3 months. Moreover, it had a better durability of effect at 6 months. In addition, there was a statistically significant improvement in fibroid-associated symptoms among both study groups but with a higher durability at 6 months among the patients of the UA group as well.
Conclusion: Our study showed that a 3-month course of UA is very effective in the management of symptomatic uterine fibroids and its results are comparable to UAE.
Keywords
Fibroid; Ulipristal acetate; Ultrasound; Uterine artery embolization; Uterine bleeding
Introduction
Fibroids are common and occur in >70% of women based on data from ultrasonography-screening studies and pathology data. However, fibroids can be asymptomatic, with clinical symptoms reported in 25–50% of women [1]. Most fibroids are asymptomatic and accidentally discovered by ultrasound done for other indications, thus require no treatment. However, they may sometimes lead to serious clinical symptoms, such as abnormal uterine bleeding (AUB), dysmenorrhea, chronic pelvic pain and pelvic pressure symptoms such as urinary frequency or urgency and constipation [2].
Ultrasonography is the preferred initial imaging modality for fibroids. Transvaginal ultrasonography (Fig. 1.) is about 90% to 99% sensitive for detecting uterine fibroids, but it may miss subserosal or small fibroids. Adding sonohysterography (Fig. 2.) or hysteroscopy improves sensitivity for detecting submucosal myomas. However, there are no reliable means to differentiate benign from malignant tumors without pathologic evaluation [2,3].
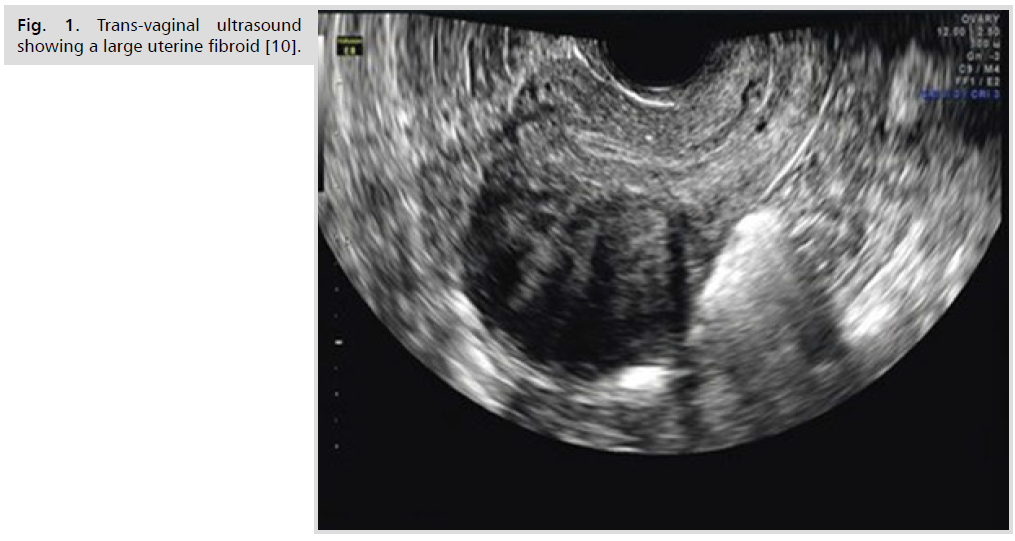
Fig 1. Trans-vaginal ultrasound showing a large uterine fibroid [10].
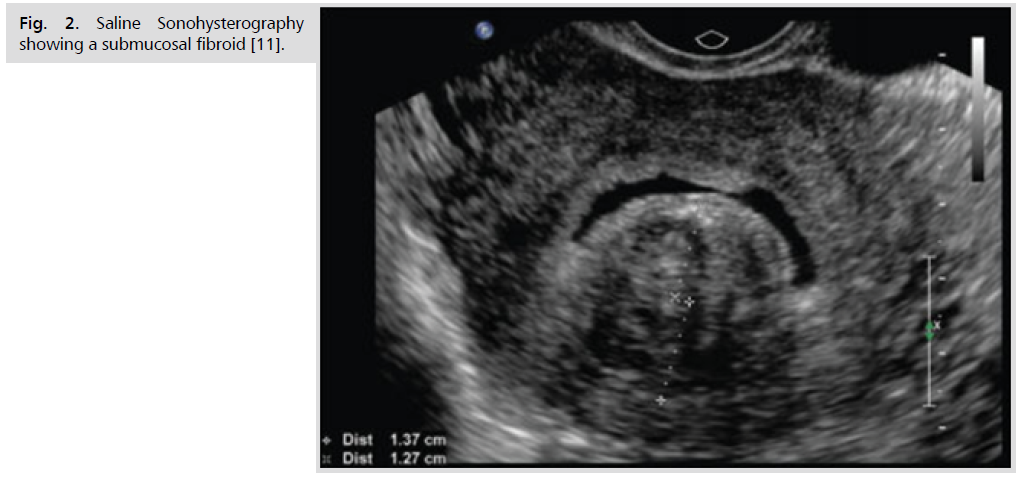
Fig 2. Saline Sonohysterography showing a submucosal fibroid [11].
The mainstay of treatment of symptomatic uterine fibroids, in literature, has always been surgical intervention in the form of either myomectomy or hysterectomy, via various routes whether abdominal, vaginal, or laparoscopic assisted. Minimally invasive alternative treatments are also used in clinical practice, such as uterine artery embolization (Fig. 3.) [3]. Nowadays, many women are seeking alternative treatment options for fibroids because of their concern that premature menopause may have significant negative impact on the future quality of life and morbidity, such as increased risk of osteoporosis, cardiovascular disease, and all are causes of mortality [4].
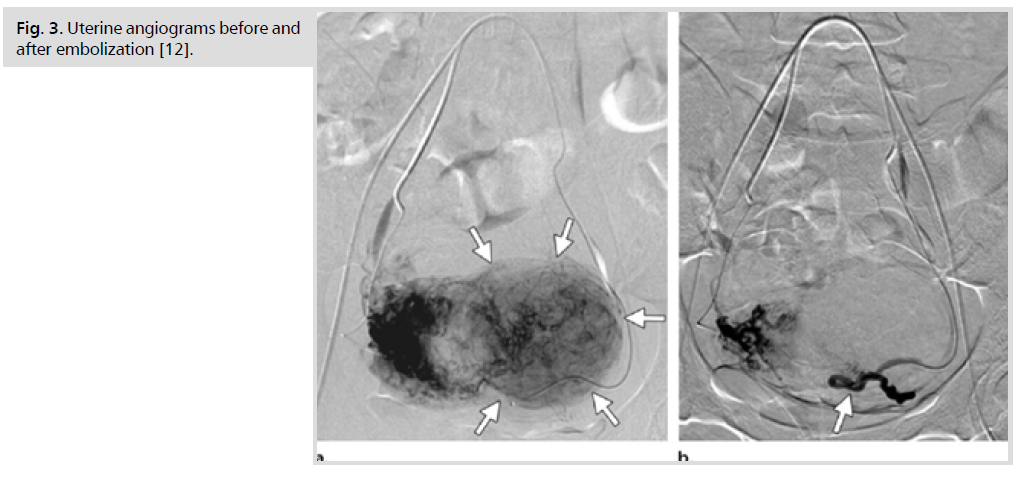
Fig 3. Uterine angiograms before and after embolization [12].
This has led to the emergence of medical therapies for uterine fibroids such as oral progestins, progestin-releasing intrauterine devices (LNG-IUS), and gonadotropin-releasing hormone (GnRH), but several studies have reported important side effects with oral progestins, such as breakthrough bleeding and increased myoma growth. Artificially induced menopause is a consequence of gonadotropin-releasing hormone treatment, the use of which is in fact approved only for short term pre-surgical therapy [5].
These intolerable side effects of the previously mentioned medical lines of treatment triggered the development of a novel line, which came out of the fact that progesterone plays an important role in stimulation of myoma growth, hence modulating its pathway with selective progesterone receptor modulators (SPRMs), such as Ulipristal Acetate (Fig. 4.), represents one new possibility for medical therapy. Thanks to its selective anti-proliferative and pro-apoptotic action, Ulipristal acetate can be effective in reducing uterine bleeding and fibroid size [6]. Ulipristal acetate also has a direct effect on the endometrium and the pituitary gland, inducing amenorrhea without a negative influence on estradiol levels or anti-glucocorticoid activity [7].
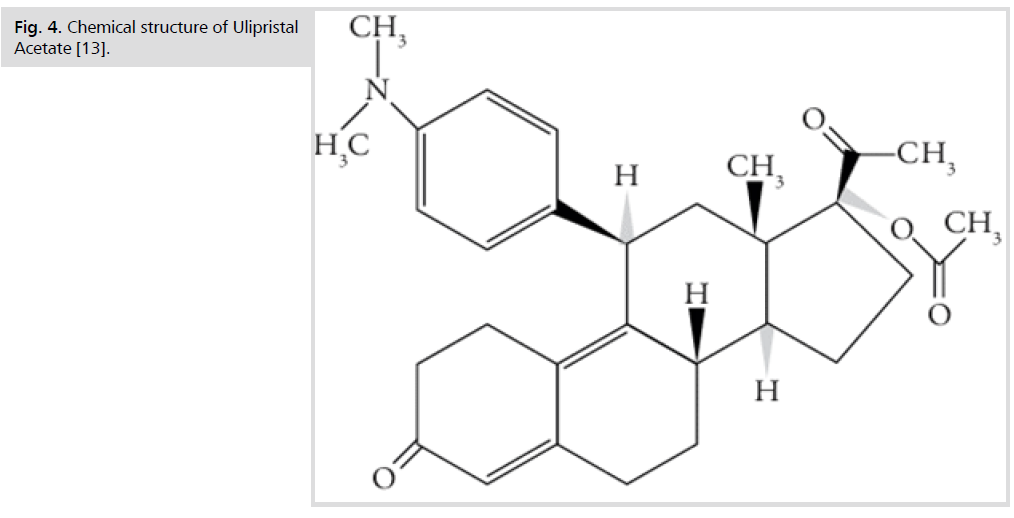
Fig 4.Chemical structure of Ulipristal Acetate [13].
Methods
This study was conducted at Ain Shams University Maternity Hospital from March 2019 to March 2021 after approval of the ethics committee of the Obstetrics and Gynecology Department. It included 70 patients, recruited from the outpatient clinic, who aged between 35 to 50 years and their BMI ranged from 18 to 35 Kg/m2. They presented with symptomatic type 2 - 6 uterine fibroids according to the FIGO classification with a dominant fibroid diameter ranging from 4 to 8 cm. All of them were not seeking fertility and refusing any surgical intervention. Post-menopausal patients or those with premature ovarian failure, patients with type 0, 1 or 7 uterine fibroids; those who underwent any previous uterine surgery or were on ongoing hormonal treatment were excluded from the study.
The nature and scope of the clinical study was explained in an understandable way to the patients and an informed consent form, in Arabic language, including all the study procedures, advantages and possible risks was provided and all the patients signed on it before participation.
All participants underwent full assessment before receiving either treatment options which included detailed history taking with personal, menstrual and contraceptive, past medical and surgical history, particularly uterine surgeries and history of present illness with detailed analysis of each patient’s complaint whether AUB, pelvic pain or pressure symptoms.
Complete physical examination was also undertaken including general examination with measurement of pulse, blood pressure, temperature, body mass index and detection of pallor due to anemia, abdominal examination for any scar or pelvi-abdominal masses and pelvic examination for uterine size, mobility and detection of palpable uterine fibroids.
Baseline investigations were also done including hemoglobin, hematocrit, blood group, AST, ALT, serum creatinine and RBS. Trans-vaginal ultrasound was performed for determination of fibroid location, using the FIGO classification system, volume and maximum diameter.
Afterwards, patients were randomized into a randomization table by closed envelops and the investigator didn’t know the order of the randomization.
They were divided into two study groups according to the randomization table:
Group A “Ulipristal Acetate (UA) - Fibristal group” (n=35):
Patients within this group received oral Ulipristal Acetate (Fibristal ©) 5 mg / day starting from the first day of menstrual bleeding, and for 3 months.
Group B “Uterine artery embolization (UAE) group” (n=35):
Patients within this group underwent bilateral selective UAE, during which polyvinyl alcohol particles were administered via a catheter followed by capping with a plug of gelatin sponge. The end point for embolization is to have a static column of contrast in the uterine artery, with only a stump filling when the internal iliac artery was injected. The gelatin sponge cap was thought to both complete the occlusion of the uterine artery and to prevent particles from being drawn out of the uterine artery by the Venturi effect, which would result in non-target embolization.
Patients within both groups underwent follow-up transvaginal ultrasound 3 months and 6 months after UA therapy or UAE session to detect the decline in fibroid volumes and diameters in comparison to their baseline. To reduce inter-observer variability associated with ultrasound imaging, measurements were always taken by the same sonographer.
Dominant fibroid volumes and diameters in patients of both study groups were assessed by ultrasound at the baseline and after 3 months of UA treatment or following UAE.
Moreover, they were clinically evaluated for symptoms reported at least 1 month before starting the treatment and 3 months after UA therapy or UAE session. Durability of the effect of treatment was tested after 6 months from initiating therapy.
Improvement of AUB was evaluated by the reduction in pictorial blood assessment chart score. This instrument is a self-administered pictorial chart that takes account of the number of sanitary pads and tampons used, presence of blood clots, and episodes of bleeding. The 1-month pictorial blood assessment chart score was calculated from the addition of daily scores for 28 days. Improvement of AUB at 3 and 6 months was detected by a score lower than 90 during the first 8 days of menstruation.
Subjective feelings of pelvic pressure, urinary urgency, and constipation was recorded on a simplified questionnaire containing 2 categories defined as “absent “and “present.” The visual analog scale pain score was used to determine the presence or absence of pelvic pain.
The study sample was a “convenient sample” where women fulfilling the inclusion criteria and consenting to participate in the study were recruited. The sample size was calculated using the PASS 15 Power Analysis and Sample Size Software (PASS©) version 15 (2017); NCSS, LLC. Kaysville, Utah, USA. A previous study reported that UA was comparable to UAE as regards the percentage of reduction in fibroid volume with median (interquartile range) percentage reduction of 48.1 (24.8 – 66) % versus 47.3 (29.5 – 69.5) % for UA and UAE respectively [7].
The required sample size has been estimated using the Power Analysis and Sample Size software version 08.0.9 (PASS;NCSS;LLC;Kaysville, Utah). The test used for calculation is the two sided z-test and type 1 error has been set at a two sided value of 0.05 (confidence level, 95%).
Using the methods described by Wan et al (2014) and Luo et al (2018), we calculated that the mean ± SD percentage of reduction in fibroid volume was 44.0 ± 28.5 % versus 48.9 ±32.3 % for UA and UAE respectively, with mean difference of 4.9 % and common within group SD of 30.5% [8,9].
It was estimated that a sample size of 35 patients per group achieves 80% power using a one-sided equivalence test with a significance level of 5% (alpha-error = 0.05), when the difference between the two means is 4.9 %, common within group SD is 30.5 %, and equivalence limits are -25% and 25%.
On the other hand, this sample size achieves a power of 82% to detect a statistically significant difference between the two groups as regards the symptoms scores and measures of ovarian reserve (AFC and AMH) for an effect size equivalent to a Cohen’s d of 0.75 (i.e., 0.75 common SD) using a two-sided unpaired t-test with the same significance level of alpha = 0.05. These equivalence limits and effect size have been targeted, as they may be considered clinically important.
Results
82 patients were assessed for their eligibility, out of which 12 were excluded as they did not meet the inclusion criteria either due to their FIGO classification (n=3), dominant fibroid diameter (n=5), previous or ongoing hormonal treatment (n=2) or previous myomectomy (n=2), leaving a total of 70 cases which were randomly allocated into the UA group (n=35) and the UAE group (n=35). There was no statistically significant difference between the baseline characteristics, such as age, BMI or parity, of the subjects of both groups as shown in Tab. 1.
| Variable | UA (n=35) | UAE (n=35) | P-value |
|---|---|---|---|
| Age (years) | 41 (37-45) | 42 (39-47) | 0.58 |
| BMI (kg/m2) | 29.6 (27.5-33.2) | 28.7 (26.1-32.9) | 0.42 |
| Parity | 2 (1-4) | 3 (2-4) | 0.11 |
Tab. 1. Baseline patients’ characteristics of both study groups. Data are presented as median (interquartile range).
In both study groups, dominant fibroid volumes and diameters showed a statistically significant decline after 3 months compared to their baseline. This decline was relatively maintained 6 months after the commencement of the study as shown in Tab. 2.
| Variable | Time | UA (n=35) (mean ± SD) |
UAE (n=35) (mean ± SD) |
p-value |
|---|---|---|---|---|
| Dominant Fibroid Volume (cm3) | Baseline | 111.7 ± 13.6 | 110.2 ± 12.4 | - |
| 3 months | 69.8 ± 11.5 | 71.2 ± 10.9 | <0.05 | |
| 6 months | 73.5 ± 12.1 | 75 ± 9.8 | - | |
| Dominant Fibroid Diameter (cm) | Baseline | 7 ± 1.02 | 7.5 ± 1.33 | - |
| 3 months | 4.5 ± 0.81 | 4.8 ± 0.87 | <0.05 | |
| 6 months | 4.9 ± 0.97 | 5.2 ± 1.11 | - |
Tab. 2. Demonstration of UA versus UAE effect on Dominant fibroid volume and diameter over 3 and 6 months using mean and standard deviation.
Thus the effect of UA therapy was quite similar to UAE regarding fibroid volume reduction after 3 months by causing about 37.5% decline in dominant fibroid volume compared to a 35.4% decline that occurred in the UAE group.
Moreover, the effect of both treatment options on the dominant fibroid diameter after 3 months in both study groups was comparable up where there was approximately 36% reduction in the fibroid diameter in both study groups.
After 6 months, the decline in fibroid volume was maintained in UA and UAE groups at 34.2% and 32% from the baseline respectively whereas the reduction in fibroid diameter in the 2 groups was 30% and 30.7% respectively as in Tab. 3.
| Variable | Time | UA (n=35) | UAE (n=35) | P-Value |
|---|---|---|---|---|
| Dominant Fibroid Volume (cm3) | 3 months | 37.5% | 35.4% | > 0.05 |
| 6 months | 34.2% | 32% | - | |
| Dominant Fibroid Diameter (cm) | 3 months | 36% | 36% | > 0.05 |
| 6 months | 30% | 30.7% | - |
Tab. 3. Percentage of decline in dominant fibroid volume and diameter in both study groups over 3 and 6 months.
Regarding fibroid-associated symptoms, as shown in Tab. 4., 42 patients presented with AUB, 20 of them were allocated in the UA group and 22 in the UAE group whereas 15 patients presented with pelvic pain out of which 7 were assigned to UA group and 8 to UAE group. Another 6 patients complained of pelvic pressure symptoms. 4 of them received Ulipristal acetate and the other 2 underwent UAE.
| Symptom | Time | UA N (%) |
UAE N (%) |
p-value |
|---|---|---|---|---|
| Heavy Menstrual Bleeding (HMB) | Baseline | 20 (57.1%) | 22 (62.8%) | - |
| 3 months | 5 (14.3%) | 7 (20%) | > 0.05 | |
| 6 months | 5 (14.3%) | 10 (28.5%) | - | |
| P- Value | - | 0.01 | 0.01 | - |
| Pelvic pain | Baseline | 7 (20%) | 8 (22.8%) | - |
| 3 months | 2 (5.7%) | 2 (5.7%) | >0.05 | |
| 6 months | 2 (5.7%) | 4 (11.4%) | - | |
| P-value | - | 0.02 | 0.02 | - |
| Pressure symptoms (Urinary & Bowel symptoms) |
Baseline | 4 (11.4%) | 2 (5.7%) | - |
| 3 months | 1 (2.8%) | 1 (2.8%) | >0.05 | |
| 6 months | 1 (2.8%) | 2 (5.7%) | - | |
| P- Value | - | 0.01 | 0.01 | - |
Tab. 4. Number and percentage of patients who showed improvement of symptoms over 3 and 6 months.
Patients of both study groups were followed up for resolution of presenting symptoms after 3 months. This follow up showed that the effect of both treatment options on fibroid-related symptoms were relatively close where UA caused relief of symptoms in 75% of patients with heavy menstrual bleeding (HMB) (n=15), and 15% of those with pelvic pressure symptoms (n=3) and 71.4% of patients who had chronic pelvic pain (n=5).
On the other hand, UAE resulted in improvement of AUB in 68.2% of patients (n=15), pelvic pain in 75% (n=6) and pressure symptoms in 50% (n=1).
In the 6-month follow-up, however, Ulipristal acetate showed a higher durability of effect on patients’ symptoms, where all patients in the UA group continued to report resolution of their initial symptoms up to 6 months after the starting point of the study.
On the other hand, 5 patients in the UAE group reported the recurrence of their symptoms and required re-intervention. Where 13% of patients with previous AUB, 25% of patients with history of pelvic pain and 50% who had pelvic pressure symptoms started to re-experience their symptoms after 6 months.
Despite the improvement of symptoms and quality of life that was recorded among both study groups, some adverse effects were also reported as shown in Tab. 5. In the UA group, headache was reported by 3 patients (8.5%), abdominal pain by 1 patient (2.8%), nausea by 2 patients (5.7%) and hot flashes by 1 patient (2.8%) However, the most serious adverse effect was hepatitis that was recorded in only one patient (2.8%).
| Adverse Effect | UA (n=35) | UAE (n=35) | P-value |
|---|---|---|---|
| Headache | 3 (8.5%) | -- | <0.05 |
| Post-procedural pain | -- | 35 (100%) | <0.05 |
| Abdominal pain | 1 (2.8%) | 6 (17.1%) | <0.05 |
| Nausea | 2 (5.7%) | -- | <0.05 |
| Hot flashes | 1 (2.8%) | 1 (2.8%) | >0.05 |
| Vaginal spotting | -- | 4 (11.4%) | <0.05 |
| Vaginal discharge | -- | 3 (8.5%) | <0.05 |
| Fatigue | -- | 1 (2.8%) | >0.05 |
| Hepatitis | 1 (2.8%) | -- | >0.05 |
Tab. 5. Number and percentage of patients who developed adverse events among both study groups.
On the other hand, no patients required re-intervention from the UA group which proves the higher durability of UA after 6 months.
In the UAE group, post-procedural pain was reported by all patients, abdominal pain by 6 patients (17.1%), vaginal spotting by 4 patients (11.4%), vaginal discharge by 3 patients (8.5%), hot flashes by one (2.8%) and fatigue by one (2.8%).
Regarding the need for re-intervention, 5 patients (14.2%) from the UAE group required re-intervention due to recurrence of symptoms after 6 months. After discussing various further treatment options which included another session of UAE or surgical intervention whether myomectomy or hysterectomy, and discussing the benefits and risks of each, 2 of these patients were scheduled for a second session of UAE, another 2 were scheduled for myomectomy and 1 patient was scheduled for hysterectomy as shown in Tab. 6.
| Type of re-intervention | Patients who required re-intervention at 6 months among UAE group (n=35) |
|---|---|
| Another session of UAE | 2 (5.7%) |
| Myomectomy | 2 (5.7%) |
| Hysterectomy | 1 (2.8%) |
Tab. 6. Number and percentage of patients in the UAE group who developed required re-intervention at 6 months.
Discussion
Principal findings of the study
The results of this study indicate that UA and UAE are equally efficacious in the treatment of fibroid-associated symptoms including AUB, pelvic pain and pelvic pressure symptoms. In fact, both treatment options caused a sonographically significant decline in dominant fibroid volume and fibroid diameter after 3 and 6 months from the start of treatment. The effect of UA was more durable that UAE as shown by the re-intervention rate in the UAE group after 6 months due to recurrence of symptoms.
On the other hand, both treatment options in the study had their drawbacks. Regarding UAE, all patients developed post-procedural pain. Other adverse effects included abdominal pain, vaginal spotting, vaginal discharge and fatigue all of which were not clinically significantly. Ulipristal acetate had similar mild adverse effects, but additionally it resulted in one warning event where 1 patient developed hepatitis which was attributed to UA-induced liver injury. Although it was reversible over few weeks after stopping the medication, this event should trigger further future studies to investigate the safety of that effective modality of treatment and minimize the probability of occurrence of such complication in the future.
Results
UA showed a statistically significant decline in dominant fibroid volume and diameter comparable to UAE at 3 and 6 months. Moreover, there was a significant improvement in fibroid-associated symptoms among both study groups with a higher durability at 6 months among the patients of the UA group.
Clinical Implications
The results of our study should encourage clinicians to implement UA in their management plan for women presenting with debilitating symptoms of uterine fibroids, but on condition that future larger multicenter randomized animal and human clinical trials with bigger sample size are done to test the safety of UA on liver function and to prevent such occurrence in patients so as to be able to safely use this medication on a wide scale in order to reduce the morbidity and mortality rates associated with other surgical and more invasive ways of treatment.
Research Implications
Larger randomized controlled trials should target the safety of use of UA on the long run. These future trials should have a larger sample size, given the low incidence of drug-induced liver injury in our study. They should also determine the cost-effectiveness of both interventions and assess their long-term positive and negative effects on patients and particularly UA. In the interim, we believe that our randomized controlled trial represents the best available evidence for consideration in guiding clinical practice.
Strengths and Limitations
The most important strengths of our study include each of the following: 1) the rigorous methodology of the study; 2) the adequate sample size and random allocation of the study subjects; 3) usage of clinically relevant inclusion and exclusion criteria; 4) the comparability of a novel modality of treatment of symptomatic uterine fibroids to a standard well-known one; 5) the remarkable similarity between the results of our study and those of previous studies in literature; 6) the study provided a longer interval of follow up for the effects of both treatment options over 6 months rather than only 3 months in other studies.
A similar study was performed in 2015 by Czuczwar et al. [7] but the follow-up, in that study, was only 3 months because UA was taken as a pre-operative medication prior to myomectomy and not as an independent modality of treatment, so longer follow-up of patients was impossible owing to the subsequent scheduled surgery.
Moreover, some limitations of our study must also be highlighted including the following: 1) did not sufficiently investigate the safety of long-term UA therapy; 2) although the follow-up was longer that the previous studies, it could have been extended to a longer interval of time; 3) Evaluation of pelvic pain was made with the use of a visual analog scale pain score in retrospect, and the definitions of urinary and gastrointestinal symptoms were made with a simplified questionnaire, as we usually done in practice [10-13].
The results of a clinical trial, by Donnez et al. [6], showed fibroid volume reductions of 21.2% in UA group and 32% in UAE group which are quite lower than ours. The authors of that study included patients with submucosal and subserosal fibroids and assessed total fibroid volume or volume of the three largest tumors by MRI, while in our study assessment was performed using ultrasound, and only the dominant fibroid volume and diameter were measured, which could explain the difference.
There is little information on the long-term effects of intermittent UA therapy, however, in another study by Donnez et al. [14], it was shown that repeated 3-month courses of UA provided effective long-term treatment for symptomatic fibroids and that concerns about its negative influence on the endometrium were not confirmed.
It must be stressed that it was our intention to compare the clinical usefulness of UA and UAE as well as their adverse effects and safety profile. To the best of our knowledge, our study is one of the first studies to also evaluate symptoms other than AUB such as pelvic pain and pressure symptoms.
Despite the proven effectiveness of UA as an independent modality of treatment for fibroids, there has been continuously increasing concerns regarding its safety particularly in long-term use. In 2012, the European Medicines Agency (EMA) approved UA 5 mg/day for the treatment of moderate to severe symptoms of fibroids in women of reproductive age, with the treatment duration limited to 3 months.
In February 2018, the EMA issued temporary restrictive measures for UA after five cases of drug-induced liver injury (DILI) were reported, four of which required liver transplantation. The Pharmacovigilance Risk Assessment Committee (PRAC) subsequently made temporary recommendations advising physicians not to take on new patients or initiate new treatment courses. In May 2018, the status of UA as a potential DILI- vinducing agent was neither confirmed nor fully ruled out; however, in eight cases of serious liver injury the role of UA was deemed possible, and the PRAC made recommendations to minimize the risk of liver injury by forbidding its use in women with underlying hepatic disorders or liver enzyme levels more than twice the upper limit of normal
To conclude, UA is an effective line of management for symptomatic fibroids by reducing fibroid volumes and its results are comparable to UAE.
Future studies should be directed towards investigating the safety of UA owing to the increasing number of reported cases of drug-induced liver injury.
We also endorse the PRAC recommendations encouraging close monitoring of liver function during and up to 4 weeks after stopping UA.
Pictoral blood assessment chart and score (Fig. 5.)
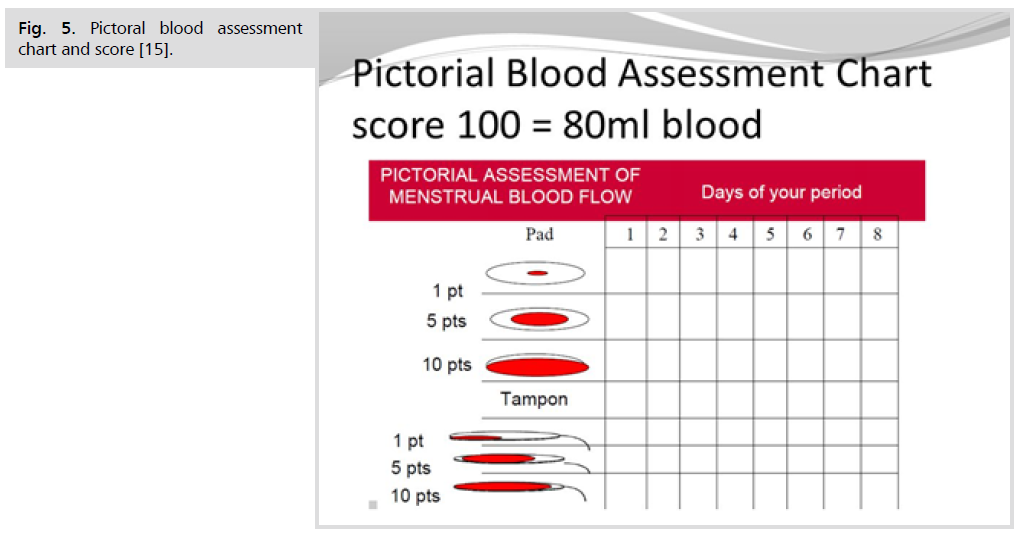
Fig 5. Pictoral blood assessment chart and score [15].
How to use the PBAC scoring system
• During the course of your period record your use of tampons and sanitary towels by placing a tally mark under the day next to the box that represents how stained your sanitary materials are each time you change them.
• Record clots by indicating whether they are the size of a 1p or 50p coin in the clots/flooding row under the relevant day. E.g. under day 1 you may say 50p × 1 and 1p × 3.
• Record any incidences of flooding by placing a tally mark in the clots/ flooding row under the relevant day.
Scores
• A lightly stained towel (PIC 1) will score 1 point, a moderately stained towel (PIC 2) 5 points, a towel which is saturated with blood (PIC 3) will score 20 points.
• A lightly stained tampon (PIC 4) will score 1 point, a moderately stained tampon (PIC 5) 5 points and a tampon that is fully saturated will score 10 points.
• A clot the size of 1p scores 1 point, a 50p sized clot scores 5 points and flooding also scores 5 points [15].
Acknowledgments
The source of funding for this study was Ain Shams University and the researchers themselves. We are also pleased to acknowledge the contributions of Dr. Yassin Eid, MD, the sonographer who performed the baseline and follow-up ultrasounds for all the study subjects and Dr. Karim Abdel Tawab, MD, the interventional radiologist who performed the uterine artery embolization procedures.
Disclosure
We, the authors, declare that we have no relevant or material financial interests with any pharmaceutical companies or other third-parties that have any relation to the research described in this paper.
Authors Contribution
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2(1):1-8.
- Hirst A, Dutton S, Wu O, et al. A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study. Health Technol Assess. (Winchester, England). 2008;12(5):1-248.
- Tropeano G, Amoroso S, Scambia G. Non-surgical management of uterine fibroids. Hum Reprod Title. 2008;14(3):259-74.
- Horak P, Mara M, Dundr P, et al. Effect of a selective progesterone receptor modulator on induction of apoptosis in uterine fibroids in vivo. Int J Endocrinol. 2012;2012.
- Lethaby A, Vollenhoven B, Sowter MC. Pre‐operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001.
- Donnez J, Tomaszewski J, Vázquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. NEJM. 2012;366(5):421-32.
- Czuczwar P, Wozniak S, Szkodziak P, et al. Influence of ulipristal acetate therapy compared with uterine artery embolization on fibroid volume and vascularity indices assessed by three‐dimensional ultrasound: prospective observational study. Ultrasound Obstet Gynecol. 2015;45(6):744-750.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):1-3.
- Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785-805.
- Allahbadia G, Merchant R. Ultrasound imaging of uterine fibroids: Evaluation and management. In Rizk B. & Puscheck E. (eds) Ultrasonography in Gynecology. Cambridge: Cambridge University Press. 2014;122-131.
- Rizk BR, editor. Ultrasonography in reproductive medicine and infertility. Cambridge university press; 2010.
- Bulman JC, Ascher SM, Spies JB. . Radiographics. 2012;32(6):1735-50.
- Talaulikar VS, Manyonda IT. Ulipristal acetate: a novel option for the medical management of symptomatic uterine fibroids. Adv Ther. 2012;29(8):655-63.
- Donnez J, Vázquez F, Tomaszewski J, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014;101(6):1565-73.
- Higham JM, O'brien PM, Shaw R. Assessment of menstrual blood loss using a pictorial chart. BJOG. 1990;97(8):734-9.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Paul Naseef*, Ayman Abou Elnour, Sherif El Mekkawi and Salma NassarCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.