Research - (2024) Volume 19, Issue 3
Relationship between premature ovarian insufficiency and bones health
Hanan Najdi Kadhim1*, Ghosoun Ghanem Kaem1 and Hameedah Hadi Abdulwahid2Received: 02-Aug-2024, Manuscript No. gpmp-24-143833; Editor assigned: 03-Aug-2024, Pre QC No. P-143833; Reviewed: 15-Aug-2024, QC No. Q-143833; Revised: 29-Aug-2024, Manuscript No. R-143833; Published: 30-Sep-2024
Abstract
Background: Premature Ovarian Insufficiency (POI) is a clinical disease characterized by an early decline in ovarian function that results in a persistent hypo-estrogenic state in women under the age of 40 years. It is distinguished by decreased ovarian follicles and insufficient ovarian sex hormones, which hasten the menopause.
Objective: Investigate the relationship between Premature Ovarian Insufficiency (POI) and bones health).
Methods: 100 women participated in a case control study,50 patients and 50 control,5ml of blood samples was collected then serum separated used to measure levels of Collagen1, Bone-specific Alkaline Phosphatase(BALP), Anti-Mullerian Hormone(AMH),Vitamin D3, serum Calcium Ca+2 using an enzyme-linked immunosorbent assay and chemiluminescent automated immunoassay system (ECL).
Results: According to results of this study there was significantly decreased in Collagen, Bone-specific Alkaline Phosphatase (BALP), Anti-Mullerian Hormone (AMH), Vitamin D3, serum Calcium Ca+2 levels in patients groups of POI) in contrast to control group.
Conclusion: There were negative effects of POI on bones health.
Keywords
Premature ovarian insufficiency (POI); Bone-specific Alkaline Phosphatase (BALP); Anti-Mullerian Hormone (AMH)
Introduction
Premature Ovarian Insufficiency (POI) is a clinical disease characterized by an early decline in ovarian function that results in a persistent hypo-estrogenic state in women under the age of 40 years [1]. It is distinguished by decreased ovarian follicles and insufficient ovarian sex hormones, which hasten the menopause [2]. About 25% of women who experience spontaneous POI may occasionally resume their ovarian activity [3]. According to estimates, at least 1% of women have POI, which can lead to long-term health issues and psychological stress. POI-related infertility was once thought to be incurable, with little to no benefit from infertility treatments. Generally speaking, it has been believed that these people cannot benefit much from treatment [4]. Alkaline phosphatase specific to bones (BSALP) BAP, a glycoprotein present on the surface of osteoblasts, is a marker of the biosynthetic activity of these cells that produce bone. It has been demonstrated that BAP is a sensitive and trustworthy marker of bone metabolism. Type 1 collagen gives the bone cells mechanical support and growth stimuli. The bone Extracellular Matrix (ECM), which is mostly made up of type I collagen and non-collagenous proteins, is essential to the process of bone remodeling. Any of these processes that are compromised might result in insufficient bone mass accumulation and maintenance, which increases the risk of osteoporosis [5]. The ovaries in females express AMH as well, and it controls the process of folliculogenesis. Serum AMH is a measure of the expanding follicle pool in clinical practice and is connected with the size of the primordial follicle pool establishing serum AMH as an accurate indicator of ovarian reserve [6].
Materials and Methods
From October 2023 to April 2024, 100 women (50 control and 50 patients) ranging age between 18 and 40 were participated in a case control study. The ethical conduct of the study was approved by the Karbala Health Directorate and the University Of Kerbala, College Of Applied Medical Sciences. After learning the objectives of the study, all patients as well as the management of the gynecological and obstetric teaching hospital gave their consent. A sample of venous blood, about 5 mL, was taken. Gel tubes were filled with the blood. After letting each blood sample clot, it was spun for ten minutes to extract the sera and conduct chemical tests for Collagen1,Bone-specific Alkaline Phosphatase(BALP), Anti-Mullerian Hormone(AMH) and Vitamin D3 using an enzyme-linked immunosorbent assay while A chemiluminescent automated immunoassay system (ECL) (Cobas e 411, Roche Diagnostic, Germany) was used to test serum Calcium Ca+2.
Inclusion criteria for the study: Fifty women between the ages of 18 and 40 who were in good health and had regular cycles were selected as the control group. They all had a history of regular menstrual cycles lasting between 28 and 33 days, no signs of thyroid malfunction, and no hyperandrogenism. Fifty women who tested positive for POI were selected as patients; all of them had received a recent diagnosis of POI from gynecologists. Each research subject provided informed consent. Additionally, each participant underwent a thorough medical investigation to define their body mass index (BMI), and characteristics like coarse facial and body hair, acne, irregular menstruation, and other disorders, and their medical history of various illnesses, gynecological disorders, and infertility.
Exclusion criteria for the study: Not a single participant in this study was older than 40 years old, nor were any of the individuals smokers. Patient with ovary cancer, autoimmune diseases, removal of ovary, females with any chronic diseases such as diabetes mellitus, heart diseases and kidney diseases, hypothyroidism patients.
Measuring of bone-specific alkaline phosphatase level
The kit measures the amount of human BALP in the sample, coats microtiter plate wells with purified humanoid BALP antibody to generate a solid-phase antibody, and then increases BALP to the wells. After thoroughly washing, the combined BALP antibody with HRP labeling became an antibody-antigen-enzyme complex. TMB substrate becomes blue upon the addition of TMB substrate solution. A sulphuric acid solution is added to halt the HRP enzyme-catalyzed activity, and the color change is detected spectrophotometrically at 450 nm. Then, by comparing the O.D. of the samples with the standard curve, the concentration of BALP in the samples is determined.
Collagen type 1 Level determination
By coating microtiters plate wells with pure Humanoid Col1 antibody, generating a solid-phase antibody, and then adding Col1 to the wells, the kit quantifies the amount of human Collagen in the sample. An antibody-antigen-enzyme-antibody complex is formed when the Col1 antibody and the HRP-labeled Col1 antibody combine. When TMB substrate solution is added following complete washing, TMB substrate turns blue. A sulphuric acid solution is added to HRP to stop the enzyme-catalyzed reaction, and the color change is detected spectrophotometrically at 450 nm. The samples' Optical Density (O.D.) is then compared to the standard curve to determine the concentration of Col1in the samples. Measuring of Anti-Mullerian Hormone Levels
By coating microtiter plate wells with purified humanoid AMH antibody, generating a solid-phase antibody, adding AMH to the wells, and combining the AMH antibody with HRP labeling to form an antibody-antigen-enzyme-antibody complex, the kit determines the amount of human AMH in the sample. The TMB substrate turns blue when the TMB substrate solution is introduced after it has been thoroughly cleaned. A sulphuric acid solution is added to halt the HRP enzyme-catalyzed activity, and the color change is detected spectrophotometrically at 450 nm. Next, by comparing the O.D. of the samples with the standard curve, the concentration of AMH in the samples is determined.
Vitamin D3 levels determination
The kit creates a solid-phase antibody by covering microtiters plate wells with cleansed humanoid VD3 antibody and quantifies the amount of human VD3 in the sample, adds VD3 to the wells, then, after carefully cleaning the plate, binds the VD3antibody with HRP labeling to produce an antibody-antigen-enzyme-antibody complex. TMB substrate becomes blue upon the addition of TMB substrate solution. A sulphuric acid solution is added to halt the HRP enzyme-catalyzed activity, and the color change is detected spectrophotometrically at 450 nm. The Optical Density (O.D.) of the samples is then compared to the standard curve to determine the concentration of VD3 in the samples.
Measuring of serum calcium levels
Total calcium content in blood, plasma, or urine can be measured using the Moorehead and Briggs-developed CPC (O-Cresol Phtalein Complexone) technology.
When calcium and CPC combine in an alkaline solution, a dark-red complex is produced, and the amount of calcium in the material is indicated by its absorbance at 570 nm.
Statistical analysis
This examination is best described as a case-control study. SPSS (Statistical Package for the Social Sciences, version 24) was utilized for statistical assessment, and an ANOVA table with the variation in data measurement that is least substantial was employed. The data is displayed as mean with Standard Deviation (SD) attached. At the (p0.05) threshold, statistical significance was considered to exist. The comparison between the groups yielded the P value, or the least significant difference.
Results
Results showed that there was significantly decreased (p<0.000) in (BALP) levels of patients group of POI (8.63 ± 4.93) in comparison to controls group (15.88 ± 3.60) (Tab. 1. and Fig. 1.), as well as results showed there was significantly decreased (p<0.000) in Collagen type 1 levels of patients group of POI (1.57 ± 0.85) in contrast to control group (2.33 ± 0.74) (Tab. 2. and Fig. 2.). The study's findings demonstrated that, compared to the control group (4.56 ± 1.09) (Tab. 3. and Fig. 3.), the patients' group with POI had considerably lower (p<0.000) levels of AMH, also there was significantly decreased ( p<0.000) in Vitamin D3 levels of patients group of POI (4.55 ± 2.12) in contrast to control group (6.39 ± 1.52), also there was significant decrease( p<0.022) in the levels of Calcium of POI group (8.21 ± 1.34) than control group (8.67 ± 0.41) (Tab. 4. and Fig. 4. and 5.).
| Sample | Mean | Std. Deviation | P Value | |
|---|---|---|---|---|
| BALP (ng/L) | Control | 15.88 | 3.60 | 0.000 |
| Patients | 8.63 | 4.93 |
Tab. 1. The average and standard deviation of BALP levels for females with and without POI.
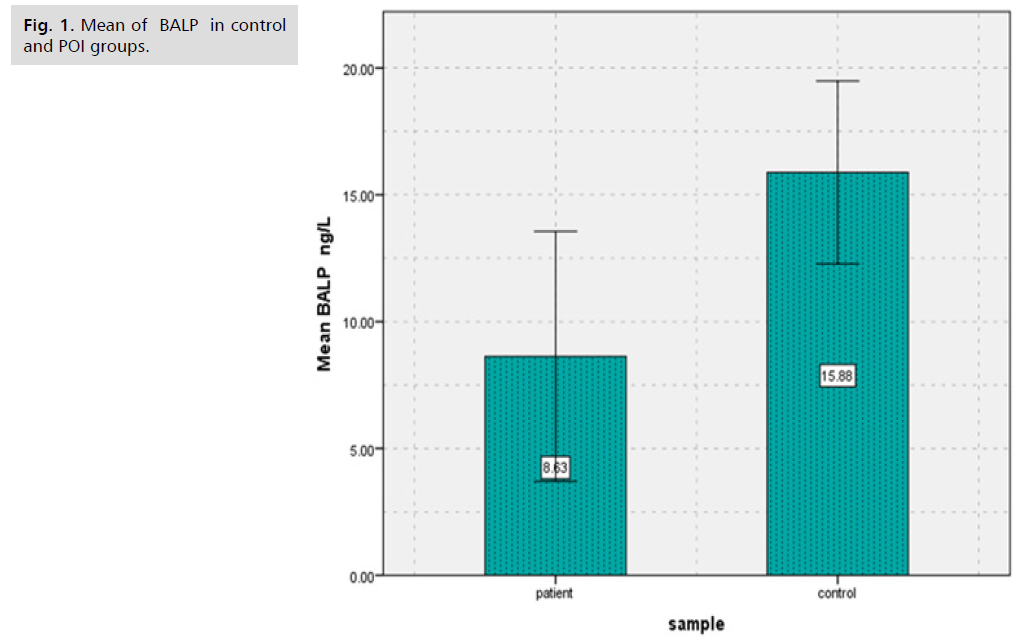
Fig. 1. Mean of BALP in control and POI groups.
| Sample | Mean | Std. Deviation | P Value | |
|---|---|---|---|---|
| Collagen type 1 (ng/L) |
Control | 2.33 | 0.74 | 0.000 |
| Patients | 1.57 | 0.85 |
Tab. 2. The average and standard deviation of collagen type 1 levels for females with and without POI.
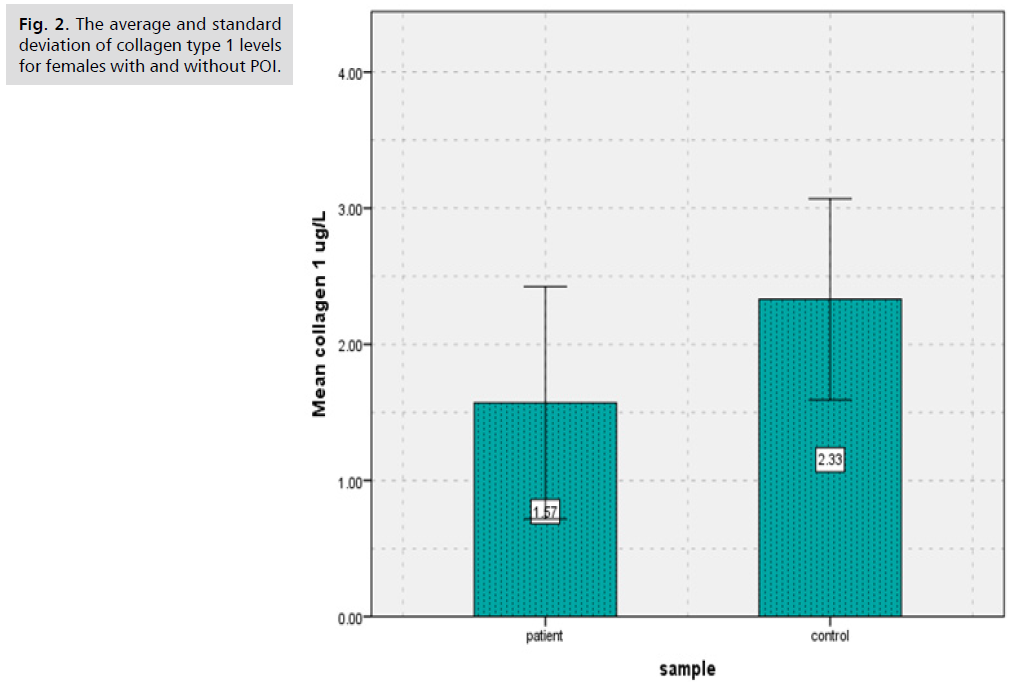
Fig. 2. Mean of collagen type 1 in control and POI groups.
| Sample | Mean | Std. Deviation | P Value | |
|---|---|---|---|---|
| AMH (ng/ml) |
Control | 4.56 | 1.09 | 0.000 |
| Patients | 2.31 | 1.03 |
Tab. 3. The average and standard deviation of (AMH) levels for women with and without POI.
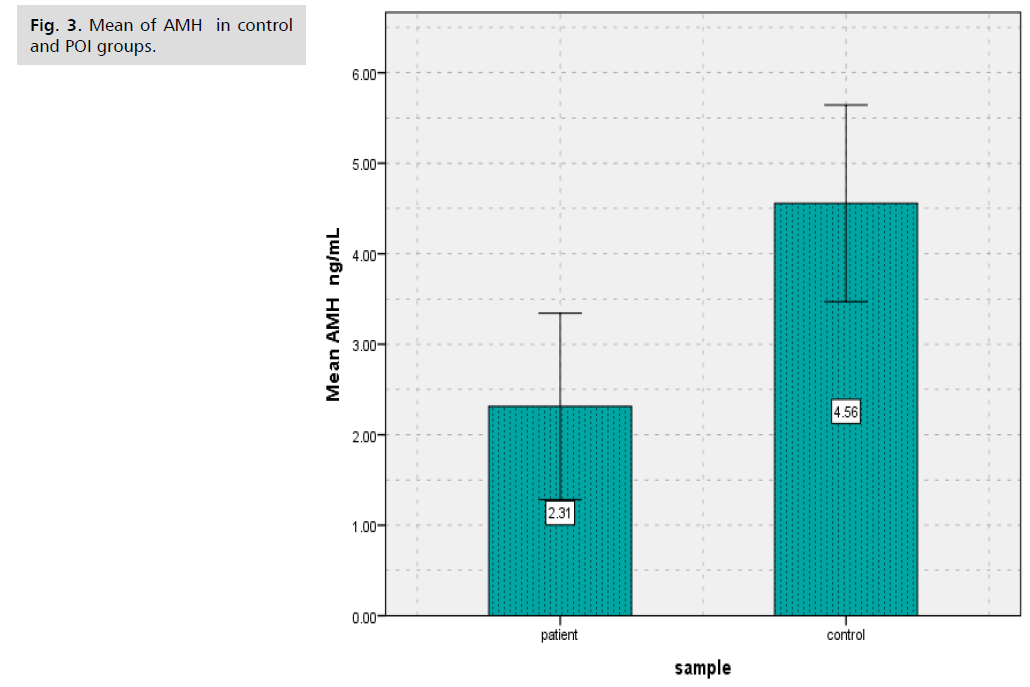
Fig. 3. Mean of AMH in control and POI groups.
| Parameters | Control group N=50 Mean |
SD | Patient Group N=50 Mean |
SD | P- value |
|---|---|---|---|---|---|
| Vitamin D3 (ng/dL) |
6.39 | 1.52 | 4.55 | 2.12 | 0.000 |
| calcium (mg/dL) | 8.67 | 0.41 | 8.21 | 1.34 | 0.022 |
Tab. 4. The average and standard deviation of vitamin D3and serum calcium levels for women with and without POI.
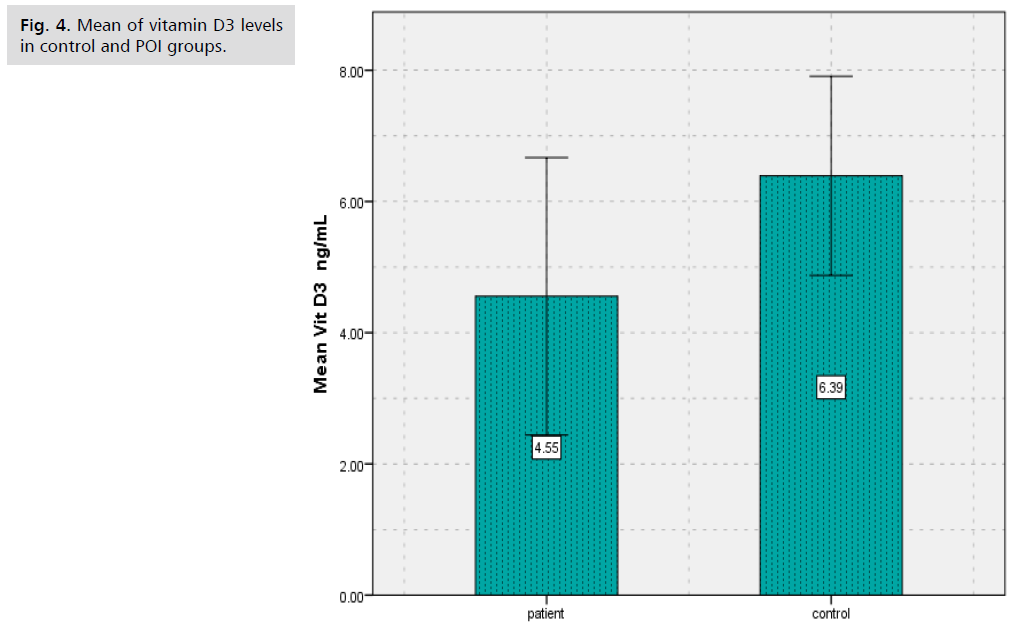
Fig. 4. Mean of vitamin D3 levels in control and POI groups.
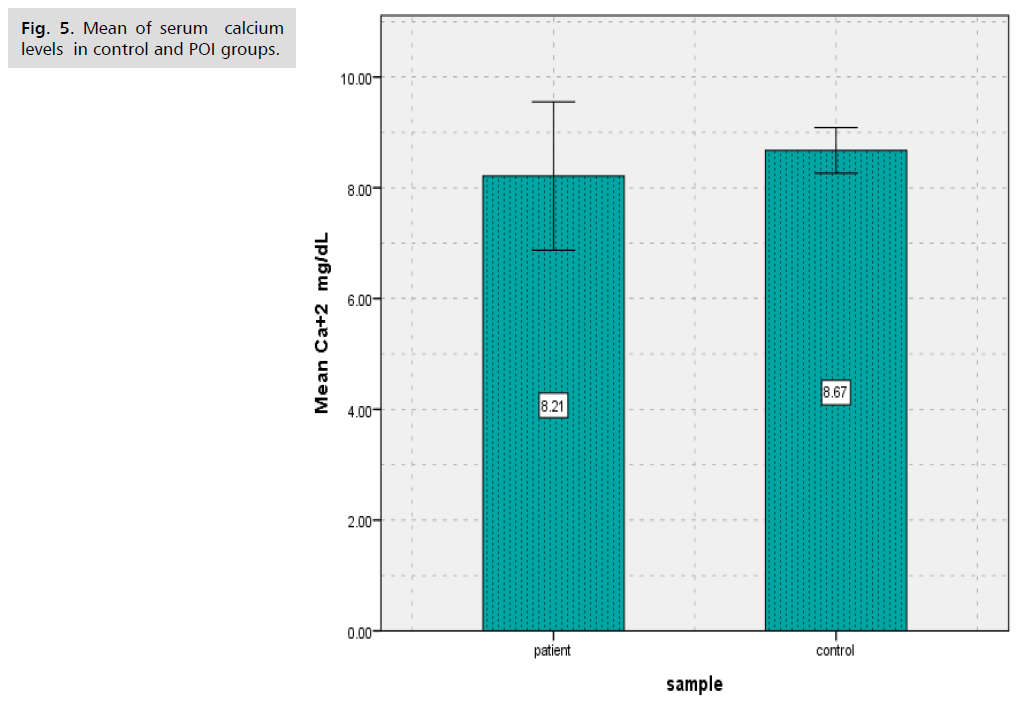
Fig. 5. Mean of serum calcium levels in control and POI groups.
Results showed that there was significantly decreased (p<0.000) in (BALP) levels of patients group of POI in comparison to controls group.
The largest quantity of bone tissue remaining at the conclusion of skeletal maturation is referred to As Peak Bone Mass (PBM). In order to reduce the chance of developing osteoporosis later in life, PBM must be attained from childhood. About 85–90% of total adult bone mass is gained by the age of 18 for girls and 20 for boys, though the age at which peak bone mass is obtained may vary depending on an individual's circumstances [7].
A decline in bone mineral density poses a threat to POI (BMD). It could be difficult for them to achieve their ideal Peak Bone Mass (PBM). The PBM attained by young girls is roughly 30% less than that of young boys. Furthermore, PBM is attained in around 50% of cases throughout puberty. Enough serum estradiol plays a major role in determining normal bone metabolism. It is anticipated that a serum estradiol level of 40 pg/ml or more is the minimal threshold needed to guarantee appropriate bone metabolism. Serum estradiol levels in untreated POI patients are known to be extremely low, typically less than 40 pg/ml. Therefore, osteopenia and osteoporosis pose a concern to POI patients [7].
Type 1 collagen is present in tiny quantities in non-skeletal tissues like skin and makes up a large portion of the bone matrix, but it is not limited to bone tissue. With the probable exception of bone, other organs do not significantly contribute to the concentration of Bone Turnover Markers (BTM) in the bloodstream since their mass is smaller than that of bone and them turnover more slowly than bone [8].
It is evident that compared to normal healthy women, women with POF typically have lower bone mineral density (BMD). As a result, BMD measurement is necessary. How frequently the tests to assess BMD should be performed is unclear at this time. To create guidelines, further prospective research is needed. Nonetheless, it would seem sensible to periodically measure BMD and to yearly check for any endocrine abnormalities in women with POF [9].
Anti-Müllerian hormone (AMH) levels in serum are frequently employed as indicators of ovarian reserve. Since AMH is created from tiny antral follicles, the quantity of primordial follicles that remain is substituted for it. POI patients reported lower levels of AMH and greater basal FSH (P<0.001) in comparison to control women [10]. Compared to control women, POI patients had higher baseline FSH and lower levels of AMH (P<0.001) [10]. In patients with POI, serum AMH levels measured using conventional techniques are extremely low or fall below the lower limit of measurement. Conversely, POI cases in cycles with tiny antral follicles may manifest very low serum AMH levels. This could help predict cycles of follicular growth and promote more effective use of limited follicle development [11].
These results were similar to the findings of a study by in Iran and another study in Baqubah, Iraq [12].
A decrease in the concentration of 25 (OH) D in serum below optimal values is known as vitamin D deficiency. This condition can potentially result in suboptimal absorption of calcium in the intestine, the development of secondary hyperparathyroidism, and an increased risk of fractures, particularly in elderly individuals. It has been demonstrated that calcium in humans can promote egg maturation either naturally or by the release of LH. This is thought to be caused by calcium modifying intracytoplasmic cAMP concentrations [13]. Sperm-derived substances also play a role in egg activation. The egg can therefore grow into an embryo. Furthermore, reproduction happens when calcium is present [14].
Discussion
Levels of some specific markers of bone health were measured in that study to investigate the effect of POI on these markers. Results showed that there was significantly decreased (p<0.000) in (BALP) levels of patients group of POI (8.63 ± 4.93) in comparison to controls group (15.88 ± 3.60), as well as results showed there was significantly decreased (p<0.000) in Collagen type 1 levels of patients group of POI (1.57 ± 0.85) in contrast to control group (2.33 ± 0.74), A decline in bone mineral density poses a threat to POI (BMD). It could be difficult for them to achieve their ideal peak bone mass (PBM). The PBM attained by young girls is roughly 30% less than that of young boys. Furthermore, PBM is attained in around 50% of cases throughout puberty. Enough serum estradiol plays a major role in determining normal bone metabolism. It is anticipated that a serum estradiol level of 40 pg/ml or more is the minimal threshold needed to guarantee appropriate bone metabolism. Serum estradiol levels in untreated POI patients are known to be extremely low, typically less than 40 pg/ml. Therefore, osteopenia and osteoporosis pose a concern to POI patients [7]. Hormone therapy has been shown to improve the bone health of women with premature ovarian insufficiency, as these individuals typically have reduced bone mineral density [15]. Approximately 94% of the organic bone matrix is composed of type I collagen. Bone remodeling and modeling happen at the same time that the skeleton develops. Since bones are not completely formed at birth, bone modeling is the gradual and ongoing process by which connective tissues builds bones until adolescence. Another ongoing process that replaces mature bone tissues with newly generated bone is called "bone remodeling." Bone turnover is another term for this process [16].
According to results of this study there was significantly decreased (p<0.000) in Vitamin D3 levels of patients group of POI (4.55 ± 2.12) in contrast to control group (6.39 ± 1.52), also there was significant decrease (p<0.022) in the levels of Calcium of POI group (8.21 ± 1.34) than control group (8.67 ± 0.41). The study's findings demonstrated that, compared to the control group (4.56 ± 1.09), the patients' group with POI had considerably lower (p<0.000) levels of AMH, evaluated serum vitamin D in POI women in a case-control study of 63 women, including 35 women with POI, in 2013 in Turkey. They reported a significant and inverse relationship between serum vitamin D level and the risk of POI (9.5 ± 4.05 ng/mL in the POI group and 18.5 ± 7.5 ng/mL in the control group; P<0.001) [17]. A tiny preventive impact against bone loss is provided by calcium supplements, but this effect vanishes when use stopped. The anti-fracture benefit of calcium supplements is restricted to elderly, fragile women or community-dwelling individuals who have low calcium intake from their diet and low vitamin D levels [18]. Serum AMH levels determined with standard methods are very low or below the lower limit of measurement in patients with POI. Very low serum AMH levels, on the other hand, may show up in POI instances in cycles with small antral follicles. This might assist anticipate cycles with follicular growth to encourage more efficient utilization of scarce follicle development [19]. One biomarker of ovarian reserve is Anti-Müllerian Hormone (AMH). Menopause between the ages of 40 and 45 may be linked to a low AMH level prior to the age of 39. Premature ovarian insufficiency (POI) is associated with a risk that is not well understood [20].
Conclusion
POI has a negative effects on bones health, there were significantly decreased levels of BALP, Collagen type 1,AMH, serum Ca+2 and vitamin D3 in women with POI.
Acknowledgement
The authors thank the College of Applied Medical Sciences, University of Kerbala for providing the necessary facilities to carry out this work.
Conflict of Interest
No conflict of interests was declared by the authors.
Funding Source
The authors did not receive any source of fund.
Data Sharing Statement
Supplementary data can be shared with the corresponding author upon reasonable request.
Authors' Contribution
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- Rahman R, Panay N. Diagnosis and management of premature ovarian insufficiency. Best Pract Res Clin Endocrinol Metab. 2021;35(6):101600.
- Wesevich V, Kellen AN, Pal L. Recent advances in understanding primary ovarian insufficiency. F1000Res. 2020;9.
- Mishra GD, Chung HF, Cano A, et al. EMAS position statement: predictors of premature and early natural menopause. Maturitas. 2019;123:82-88.
- Golezar S, Ramezani Tehrani F, et al. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019;22(4):403-411.
- Manocha A, Kankra M, Singla P, et al. Clinical significance of reproductive hormones. Current Medicine Research and Practice. 2018;8(3):100-108.
- Di Clemente N, Racine C, Pierre A, et al. Anti-Müllerian hormone in female reproduction. Endocrine Reviews. 2021;42(6):753-782.
- Meczekalski B, Niwczyk O, Bala G, et al. Managing early onset osteoporosis: the impact of premature ovarian insufficiency on bone health. J Clin Med. 2023;12(12):4042.
- Brown JP, Don-Wauchope A, Douville P, et al. Current use of bone turnover markers in the management of osteoporosis. Clin Biochem. 2022;109:1-0.
- Takahashi A, Yousif A, Hong L, et al. Premature ovarian insufficiency: pathogenesis and therapeutic potential of mesenchymal stem cell. J Mol Med. 2021;99(5):637-650.
- Wang C, Sun Y. Induction of Collagen I by CXCL10 in Ovarian Theca–Stroma Cells via the JNK Pathway. Front Endocrinol. 2022;13:823740.
- Vimalraj S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene. 2020;754:144855.
- Al-Hamdany WA, Gaffar MK. A comparison effect of dysmenorrhea and secondary amenorrhea in the concentration of some biochemical and some hormones in women in Tikrit City. Tikrit J Pure Sci. 2019;24(2):37-42.
- Ahuja A, Parmar D. Role of minerals in reproductive health of dairy cattle: a review. Int J Livest Res. 2017;7(10):16-26.
- Chen Q, Ke H, Luo X, et al. Rare deleterious BUB1B variants induce premature ovarian insufficiency and early menopause. Hum Mol Genet. 2020;29(16):2698-2707.
- Costa GP, Ferreira-Filho ES, dos Santos Simoes R, et al. Impact of hormone therapy on the bone density of women with premature ovarian insufficiency: a systematic review. Maturitas. 2023;167:105-112.
- Rani S, Bandyopadhyay-Ghosh S, Ghosh SB, et al. Advances in sensing technologies for monitoring of bone health. Biosensors. 2020;10(4):42.
- Dashti S, Tabrizi AT, Maleki ZF, et al. Relationship between fat-soluble vitamins and premature ovarian failure: A systematic review. J Nurs Midwifery Sci. 2023;10(3).
- Heidari B, Hajian-Tilaki K, Babaei M. Effectiveness and safety of routine calcium supplementation in postmenopausal women. A narrative review. Diabetes Metab Syndr. 2020;14(4):435-442.
- Iwase A, Osuka S, Goto M, et al. Clinical application of serum anti‐Müllerian hormone as an ovarian reserve marker: A review of recent studies. J Obstet Gynaecol Res. 2018;44(6):998-1006.
- Desongnis S, Robin G, Dewailly D, et al. AMH assessment five or more years after an initially low AMH level. Eur J Obstet Gynecol Reprod Biol. 2021;256:70-74.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Hanan Najdi Kadhim1*, Ghosoun Ghanem Kaem1 and Hameedah Hadi Abdulwahid22Department of Obstetrics and Gynecology, College of Medicine, University of Warith Al-anbyiaa, Kerbala, Iraq
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.