Research Article - (2024) Volume 19, Issue 4
Received: 02-Sep-2024, Manuscript No. gpmp-24-147090; Editor assigned: 03-Sep-2024, Pre QC No. P-147090; Reviewed: 14-Oct-2024, QC No. Q-147090; Revised: 29-Nov-2024, Manuscript No. R-147090; Published: 30-Dec-2024
In this research, a green nanocomposite was meticulously prepared and characterized. Its biological effectiveness was thoroughly evaluated before and after loading it with metformin in female white rats induced with polycystic ovary syndrome (PCOS). The green nanocomposite, derived from fresh, soft asparagus stalks, underwent rigorous preparation and was carefully characterized. The nanocomposite was meticulously created by mixing 20% fresh asparagus plant with 80% silver nitrate solution (AgNO3), and then the treatment was loaded onto it and diagnosed. Rigorous analysis involving Fourier transform infrared diffraction spectroscopy (FT-IR) and Atomic Force Microscope (AFM) images contributed to unveiling significant changes in the nanocomposite surface loaded with the treatment, all within nanoscale sizes and dimensions consistent with the results of the infrared spectrum (FT-IR). The therapeutic treatments' impact on immune parameters was comprehensively studied using 25 female white rats divided into five groups. The study conclusively indicated a significant increase (P<0.05) in the levels of cytokines IL-10 and IL-17 in the PCOS induction group (G2) compared to the control group (G1). Additionally, a significant decrease (P<0.05) in the levels of cytokine IL-10 in all treatment groups (G3-Meto., Asp.NPs-G4, G5- Asp.NPs+Meto.) compared to the PCOS induction group (G2), and a decrease (P<0.05) in the level of IL-17 in all treatment groups (G3-Meto., Asp.NPs-G4, G5- Asp.NPs+Meto.) compared with the PCOS induction group (G2) were noted. The evidence was compelling and led to the conclusion that the nano-asparagine complex was successfully prepared and displayed superior effectiveness in treating polycystic ovary syndrome compared to metformin. Furthermore, when metformin is loaded onto the nano-asparagine complex, its therapeutic effectiveness increases, reducing treatment time, side effects, and financial costs associated with repeated treatment.
Preparation of green nanocomposites; Diagnostic tests for nanocomposites; Metformin drug; Fresh green Asparagus Officinalis L. stems; Polycystic ovary syndrome
The process of synthesizing nanoparticles using plants offers several advantages over other biological methods. It eliminates the need to maintain cell density, prevents contamination from other microbial growth, and is a simple one-step synthesis method without the risk of mutations found in microorganisms. Additionally, the extraction and separation process can be easily scaled up for large-scale synthesis of nanoparticles [1,2]. Compared to microorganisms, producing plant extracts tends to be less expensive [3]. The green synthesis of nanoparticles using plant compounds as bioreducers is gaining momentum, with various plant materials such as leaf, fruit, bark, fruit peel, root, and callus extracts being utilized [4,5]. The extract contains flavonoids, terpenoids, and alkaloids that are said to help reduce and stabilize nanoparticles [6,7]. The use of nanoparticles in drug delivery technology offers several advantages. These include the ability to easily control the size and surface properties of nanoparticles to effectively deliver drugs to specific targets in the body, thereby increasing therapeutic efficiency while reducing side effects. Additionally, nanoparticles allow for controlled drug release and enable more accurate and rapid targeting of specific sites [8,9].
Polycystic Ovary Syndrome (PCOS) Studies have shown that about 8-13% of women of reproductive age and about 3-11% of adolescent girls suffer from PCOS. This syndrome is a chronic, complex, multisystem disorder and is one of the most well-known endocrine disorders that causes constant anxiety for affected women [10,11]. PCOS is a diverse disorder with different health effects and clinical symptoms that can appear in childhood and change as a person enters adolescence and adulthood [12]. There are numerous significant consequences of PCOS, including obesity, metabolic syndrome, impaired glucose tolerance, type 2 diabetes, hirsutism, acne, cardiovascular risk factors, anovulation, fertility issues, pregnancy complications, depression, anxiety, eating disorders, and a reduced quality of life [13-19]. PCOS also imposes a substantial financial burden. In the United States, the annual cost of treating the many associated health issues, such as hirsutism, infertility, diabetes, and obesity, is estimated to be approximately $4 billion [20,21].
Metformin, also known as 1,1-dimethylbiguanide hydrochloride, is an oral hypoglycemic drug used to treat type 2 diabetes [22,23], polycystic ovary syndrome (PCOS) [24], and other conditions such as insulin resistance in the liver. It works by inhibiting hepatic glucose production and increasing glucose uptake and utilization in peripheral tissues, such as muscle cells [25-28]. Metformin has the potential to reduce body weight [22,29] and induce ovulation [22,30]. Moreover, studies have suggested that metformin inhibits steroidogenesis in the ovary and reduces androgen production from the ovarian theca cells [22,31,32]. This makes it an important drug for the treatment of PCOS, even though the treatment process may take a considerable amount of time. There is also a good physiological basis to believe that by suppressing insulin levels through the use of insulin-sensitizing agents like metformin, it can alleviate the negative effects of ovarian stimulation and improve treatment outcomes such as ovulation and pregnancy rates [25,27,33]. Additionally, metformin may also act directly on ovarian cyst cells, reducing androgen production [25,32,34].
Cytokines are a diverse group of small proteins, typically ranging from 5-20 kDa in size [35]. They act as regulatory proteins and are secreted by cells of the immune system. These soluble, low molecular weight proteins play a crucial role in modulating the balance of humoral and cellular immunity. Additionally, they are involved in a wide range of biological activities, particularly in regulating and activating the growth, differentiation, and activation of immune cells [36,37]. Cytokines are produced by various immune cells, including B-lymphocytes, activated T-lymphocytes, macrophages, mast cells, natural killer (NK) cells, fibroblasts, and endothelial cells. It's important to note that cytokines are distinct from hormones or growth factors and function differently compared to hormones. These proteins are usually produced by specific cells [36]. Cytokines exert their effects by binding to specific receptors on target cells. The expression of these receptors on the target cells determines the amount and type of response induced by the cytokines [38]. The immune system consists of various pathways that involve pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1, IL-2, IL-6, IL-8, IL-12, and IL-17, as well as anti-inflammatory cytokines like IL-4, IL-10, and TGF-β. These cytokines play a crucial role in the development of autoimmune diseases. The balance between pro-inflammatory and anti-inflammatory cytokines in the immune system determines how T-cells respond during an immune reaction. Pro-inflammatory cytokines contribute to the onset and spread of autoimmune inflammation, whereas anti-inflammatory cytokines aid in reducing inflammation and promoting recovery from the acute phase of the disease. This process is influenced by the interaction between T-helper 1 and T-helper 2, and has been a major focus in studies on cytokines and autoimmunity [39,40].
Synthesis of silver nanoparticles using aqueous extract of fresh asparagus stems
Silver nanoparticles were synthesized by dissolving 1 millimolar of silver nitrate in 100 ml of non-ionic water at a temperature of 80 °C. The aqueous extract was prepared by dissolving 2.5 g of dried asparagus extract in 100 ml of non-ionic distilled water. Next, 20 ml of the asparagus extract was added to 80 ml of the dissolved silver nitrate solution, with a volume ratio of 80:20 (silver nitrate solution: plant extract) and at a pH of 7. The color change was observed, and the glass tubes were covered with thin silicone paper and kept in the dark in a refrigerator for five days until the color stabilized. The stabilized solution was then used in the experiment and dosed to the experimental animals once a day [41].
Glucophage drug (Metformin)
Metformin is produced by Merck Sante s.a.s2, rue du Pressoir Vert-45400 SEMOY-France. Each box contains 5 strips, with each strip containing 20 tablets and each tablet containing 500 mg. This 500 mg is one of the therapeutic doses used for humans. The required therapeutic dose was prepared in the study based on the equation for converting the therapeutic dose from humans to animals, as calculated according to [42].
Loading the drug metformin on the green nano-asparagus compound
To load the drug metformin onto the green nano-asparagus compound, start by dissolving 1250 mg of metformin in 40 ml of deionized water on a hot plate magnetic stirrer. Then, take a graduated glass beaker and add 40 ml of the green nano-complex to it. Next, add the dissolved metformin to the beaker. Cover the beaker with silicone paper and leave it on the hot plate magnetic stirrer (not exceeding 50 degrees Celsius) until the mixture reaches a volume of 13.33. After reaching this volume, add more of the green nano-complex until the volume reaches 40 ml again. Return the mixture to the device for 10 minutes to ensure homogeneity, and then store it in the refrigerator until it is used in the experiment [43,44].
Study groups
The study involved 25 female white rats aged 5-7 months, weighing between 165-175 grams. The rats were randomly divided into four groups for two treatment periods, with each group comprising five females. The groups were treated as follows: Negative control group (G1): Five female white rats were dosed with physiological saline (0.9%), i.e., normal saline only. Positive control/induction group (G2): Five female white rats were orally dosed with 1mg of letrozole dissolved in 1% Carboxymethylcellulose powder (CMC) dissolved in 99% distilled water for 28 days to induce polycystic ovary syndrome. Group treated with metformin only (G3): Five female albino rats with induced polycystic ovary syndrome were treated with oral metformin at a concentration of 500 mg/kg body weight for 28 days. Group treated with nano-asparagine only (G4): Five female rats with induced polycystic ovary syndrome were treated with oral doses of nano-asparagine at a concentration of 300 mg/kg of body weight for 28 days. Group treated with nano-asparagine loaded with metformin (G7): Five female albino rats with induced polycystic ovary syndrome were treated with oral doses of nano-asparagine loaded with metformin at a concentration of 300 mg/kg of body weight for 28 days.
Collection of blood samples
Blood samples were taken from the animals that were both treated and untreated after being induced with the disease. Each animal's blood, drawn directly from the heart by heart puncture after being anesthetized with chloroform, was collected at a rate of 3 ml using sterile medical syringes and placed in gel tubes without any anticoagulant. The tubes were left for 15-20 minutes at room temperature for the blood to clot before being centrifuged at 3000 rpm for 5-10 minutes to separate the blood serum. The sera were transferred to 1.2 ml Eppendorf tubes and stored in the refrigerator at 20 °C to conduct immune tests, which included assessing inflammatory and anti-inflammatory interleukins (IL-10 and IL-17) [45].
Immune tests estimation
Method of measuring IL-17 and IL-10 concentration
Identification of metformin, asparagus stem extract and asparagus nanocomposite before and after loading with metformin.
Infrared Spectroscopy (FT-IR)
FT-IR is a crucial tool for identifying active functional groups. The FT-IR spectra of AgNPs synthesized using an aqueous extract of fresh green asparagus stalks are provided, indicating the groups responsible for covering and stabilizing the nanoparticles [46]. These techniques are used to study the secondary structure and assembly of proteins, as well as the composition of carbohydrates and nucleic acids. The resulting curves show the chemical groups and help identify the active compounds in the extract that contribute to the oxidation and reduction processes involved in producing nanoparticles [47]. To identify the active functional groups in the aqueous extract of fresh green asparagus stalks responsible for reducing silver nitrate to silver nanoparticles and stabilizing them, an infrared spectrometer equipped with Fourier transform (FTIR) should be used. The FTIR analysis results, shown in Fig. 1. 2. and 3., revealed different bond extensions at various wave numbers. The AgNPs synthesized using the aqueous extract of fresh green asparagus stalks exhibited overlapping bands ranging between 1076.32-1500 cm-1. In contrast, the metformin drug displayed bands at frequencies of 934.91-1497.82 cm-1. When examining the nano asparagus complex loaded with metformin drug, we observed a shift in frequencies to 2074.85, 3437.20, and 1637.71 cm-1, indicating the presence of stretching of the hydroxyl groups O-H bonds found in alcohols and phenols.
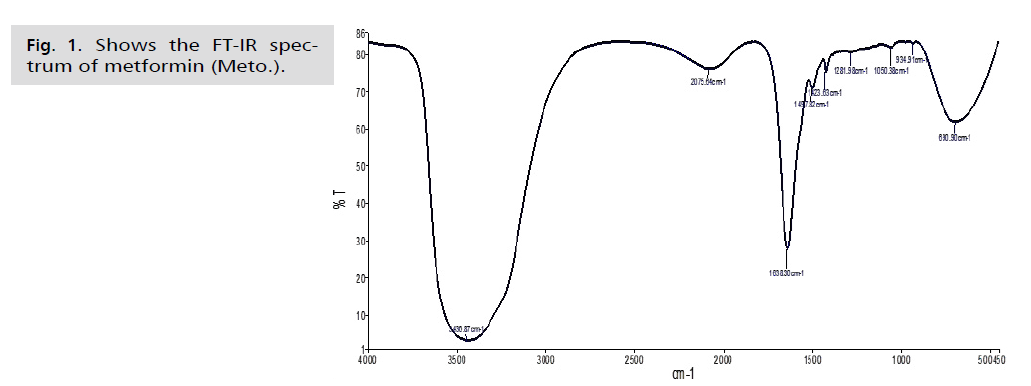
Fig. 1. Shows the FT-IR spectrum of metformin (Meto.).
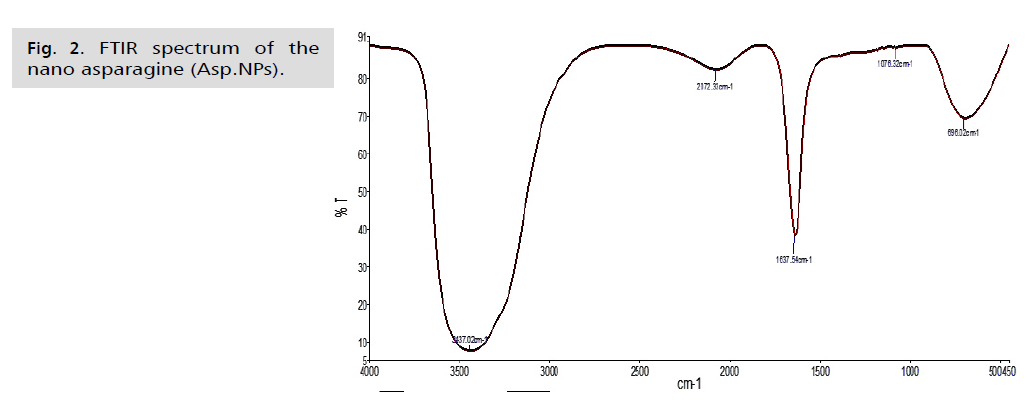
Fig. 2. FTIR spectrum of the nano asparagine (Asp.NPs).
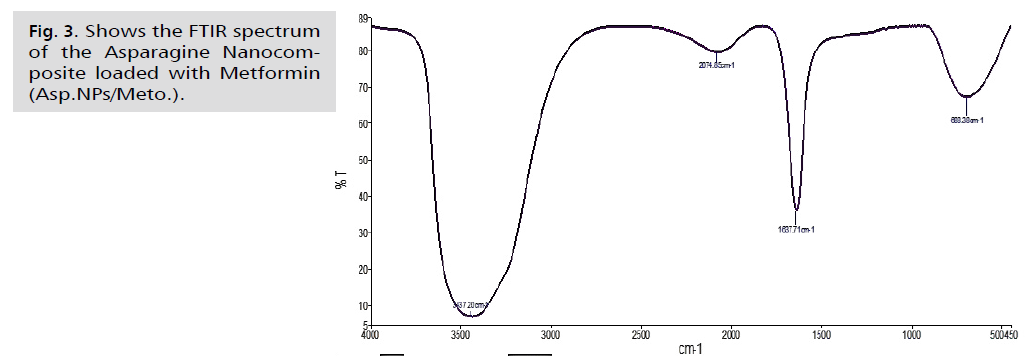
Fig. 3. Shows the FTIR spectrum of the Asparagine Nanocomposite loaded with Metformin (Asp.NPs/Meto.).
The AgNPs composite silver nanoparticles showed a broad absorption peak at a frequency of 3437.02 cm-1, which is attributed to the N-H amine group stretching vibration. In comparison, the free metformin drug exhibited an absorption peak at a frequency of 3436.87 cm-1, also indicating the N-H amine bond stretching vibration. The infrared spectrum of the drug-loaded nanocomposite displayed a shift towards a frequency of 3437.20 cm-1, suggesting the N-H amine bond stretching. Furthermore, in the nano-asparagine complex, the bands appeared at frequencies between 1000 - 1500 cm-1, indicating the vibration of the C-O bond, which exhibits a strong extension. In the analysis of the metformin drug, we observed frequency bands between 1050.38-1497.82 cm-1, which are attributed to the C-N bond and exhibit strong extension. When examining the nano-composite loaded with the drug, we noticed a shift of the band at 1637.71 cm-1compared to the examination of the nano-asparagine complex and the metformin drug. However, the band at 2074.85 cm-1 remained at almost the same frequencies. The less broad peak of 1637.54 cm-1 in the asparagine nanocomposite test indicates the extension of the C=C bond in the alkene group. This differs from the free drug test, in which the peak of 1638.30 cm-1resulted from the C=C bond. In the test of the asparagine nanocomposite loaded with the drug, we observed that the peak remained at the same frequency of 1637.71 cm-1. The infrared spectrum of the nanocomposite loaded with the drug showed a shift and stretching of the C-O bond in the frequency range of 1000-1500 cm-1, following the results of the test for the asparagine nanocomposite and the metformin drug test, which were at the frequencies of 1050.38 and 1076.32 cm-1, respectively. The low peaks observed in the nano-asparagus, drug, and drug-loaded nanocomposite at frequencies of 690.90, 696.02, and 688.38 cm-1, respectively, within the C-H bond, showed strong stretching of alkanes. There was a noticeable shift in the results between the drug and drug-loaded nanocomposite examinations. The vibrational peaks (absorption and transmittance) were consistent with the presence of flavonoids, proteins, and terpenoids in the aqueous extract of fresh green asparagus stems. These compounds are likely responsible for the synthesis and reduction of nanoparticles, as reported by Posinastty, et al. [48] and Rojas and Asmat-Campos [49]. The presence of organic functional groups, such as alkanes and alcohols, played an important role in reducing silver nitrate and forming silver nanoparticles. These groups also helped prevent the particles from oxidation and deterioration, contributing to their long-term stability [50,51]. The displacements observed in the drug-loaded nanocomposite provide clear evidence of metformin being loaded onto the asparagine nanocomposite.
Atomic Force Microscopy (AFM)
In Fig. 4. and 5., the current study presented microscopic images captured by the atomic force microscope. The roughness coefficient of the outer surface of the asparagus nanocomposite particles was 0.99681 nm, while the surface roughness coefficient of the aqueous extract of fresh green asparagus stalks was 1.42235 nm. The difference before and after converting the aqueous extract of fresh green asparagus stalks to the nanocomposite was 0.42554 nm, signaling the success of the process of preparing the nanocomposite from the aqueous extract of green asparagus. In comparison, the roughness coefficient of the free metformin drug was 1.9006 nm. Furthermore, the square root rate of the asparagus nanocomposite was 1.28600 nm, whereas the aqueous extract of fresh green asparagus stalks measured 1.84616 nm. The difference in the square root rate was 0.56016 nm, implying that the nanocomposite falls within the nanoscale limits [43]. The square root of the free Metformin was 2.36988 nm. The surface area of the asparagus nanocomposite was 3.113%, and the surface area of the aqueous extract of fresh green asparagus stalks was 5.019%. This indicates that the surface is a good receptor for the particles to be loaded. The surface area of the free metformin was 7.441%. The maximum peak height of the asparagus nanocomposite was 9.9939, and the maximum peak height of the aqueous extract of fresh green asparagus stalks was 11.7714. The maximum peak height of the free metformin was 13.6780. The maximum pit height of the asparagus nanocomposite was 4.5740, and the maximum pit height of the aqueous extract of fresh green asparagus stalks was 11.4198. The maximum pit height of the free metformin was 10.9336. The surface luminous intensity values of the asparagus nanocomposite were 2.186, and the surface luminous intensity values of the fresh green asparagus extract were 2.857. The surface light intensity values of free metformin were 3.531. The study's results revealed that the average diameter of the Asp.NPs nano asparagus compound particles was 45.83 nm. This was in contrast to the average diameter of the green asparagus extract Asp. particles, which measured 116.9 nm. The difference between the two average sizes was 71.07 nm. Additionally, the average diameter of the free metformin particles was 176.5 nm, as indicated in Tab. 1.Fig. 6. of the study showed that the nano-asparagine complex loaded with metformin had a roughness coefficient of 3.03736 nm, while the roughness coefficient of free metformin was 1.90062 nm. The difference in the roughness coefficient was 1.13674 nm. This is a significant finding as it ensures that the nano-asparagine complex is properly loaded with metformin following the conversion of fresh green asparagus into the nano-complex [52]. The square root mean of the nano-asparagine complex loaded with metformin was 4.34698 nm, while that of metformin alone was 2.36988 nm, with the difference in the square root mean being 1.9771 nm. Additionally, the surface area of the nano-asparagine complex loaded with metformin was 27.80%, whereas the surface area of free metformin was 7.441%. The results also indicated that the surface luminescence intensity values of the metformin-loaded nano-asparagine were 3.793, while those of the free metformin were 3.531. Furthermore, the maximum peak height of the metformin-loaded nano-asparagine was 27.6571, in contrast to the 13.6780 value of the free metformin. The maximum pit height of the metformin-loaded nano-asparagine was 23.6796, while that of the free metformin was 10.9336. The average particle size of the metformin-loaded nano-asparagine Asp.NPs/Meto. Was 278.4 nm. This is larger than the average particle size of metformin alone, which was 176.5 nm, and the nano-asparagine, which was 45.83 nm. This demonstrates the successful loading of metformin. This information is summarized in Tab. 1., which illustrates the physical properties of metformin particles, fresh green asparagus stem extract, and the asparagus nanocomposite before and after loading with metformin.
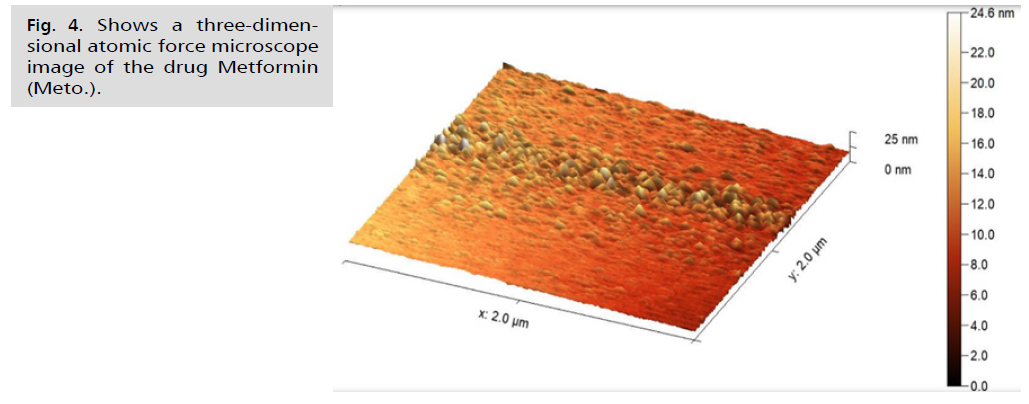
Fig. 4.Shows a three-dimensional atomic force microscope image of the drug Metformin (Meto.).
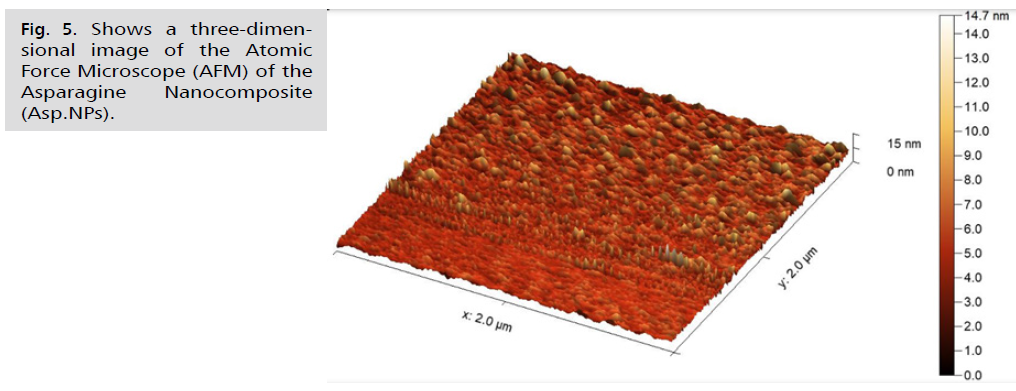
Fig. 5. Shows a three-dimensional image of the Atomic Force Microscope (AFM) of the Asparagine Nanocomposite (Asp.NPs).
| Physical properties of therapeutic agents | Meto. (nm) | Asper. (nm) | Asper.Nps (nm) | AsperNps /Meto. (nm) |
|---|---|---|---|---|
| Maximum pit height (Sv) | 10.9336 | 11.4198 | 4.7540 | 23.6796 |
| Kurtosis (Sku) | 3.531 | 2.857 | 2.186 | 3.793 |
| Developed interfacial area ratio (Sdr) | 7.441% | 5.019% | 3.113% | 27.80% |
| Maximum peak height (Sp) | 13.6780 | 11.7714 | 9.9939 | 27.6571 |
| Arithmetical mean height (Sa) | 1.90062 | 1.42235 | 0.99681 | 3.03736 |
| Root mean square height(Sq) | 2.36988 | 1.84616 | 1.28600 | 4.34698 |
| Mean size | 116.9 | 176.5 | 45.83 | 278.4 |
Tab. 1. Physical properties of metformin, asparagus stem extract and green asparagus nanocomposite before and after loading with metformin.
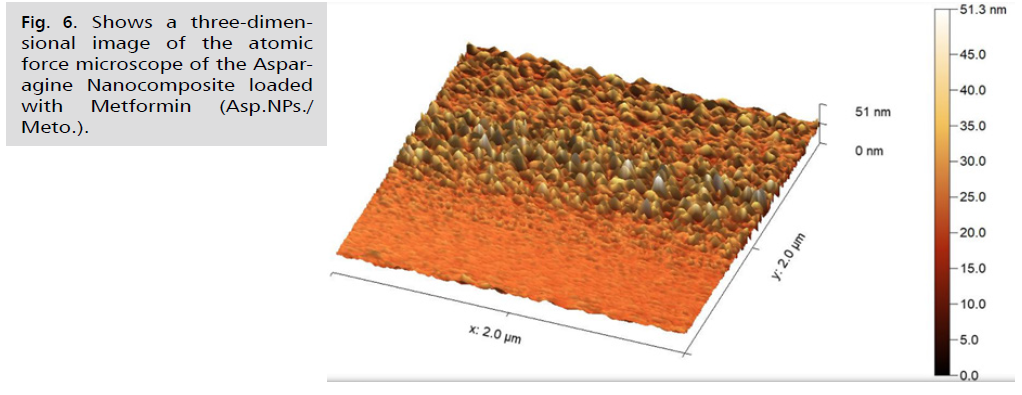
Fig. 6. Shows a three-dimensional image of the atomic force microscope of the Asparagine Nanocomposite loaded with Metformin (Asp.NPs./Meto.).
Study the effect of induction of polycystic ovary syndrome and therapeutic treatments on some immune parameters (IL-10) and (IL-17) in the blood serum of female rats
The results of the current study, as shown in Tab. 2., revealed different levels of immune parameters in the various groups. It is important to note that there was a significant increase (P<0.05) in the level of IL-10 in the group with induced PCOS (G2) compared to the control group (G1). Additionally, there was a significant increase (P<0.05) in the level of IL-10 in the group treated with Meto (G3) compared to the control group (G1). Furthermore, the results indicated a significant increase (P<0.05) in the level of IL-10 in the group treated with Asp.NPs. (G4) compared to the control group (G1). In contrast, the group treated with Asp.NPs./Meto (G5) showed an increase in the level of IL-10 that did not reach statistical significance compared to the control group (G1). The results from Tab. 1. also demonstrated a decrease in the level of IL-10 in the group treated with Meto. (G3) compared to the PCOS induction group (G2), although it did not reach statistical significance. Moreover, there was a significant decrease (P<0.05) in the level of IL-10 in the group treated with Asp.NPs. (G4) compared to the PCOS induction group (G2). Similarly, there was a significant decrease (P<0.05) in the level of IL-10 in the group treated with Asp.NPs./Meto (G5) compared to the PCOS induction group (G2).
| Transactions | IL-10 | IL-17 |
|---|---|---|
| Control | 0.613 ± 0.095 | 0.146 ± 0.740 |
| PCOS | 1.320 ± 0.146 | 0.101 ± 1.213 |
| Meto. | 1.053 ± 0.309 | 0.131 ± 1.048 |
| Asp.NPs | 0.820 ± 0.096 | 0.097 ± 0.941 |
| Asp.NPs/ Meto. | 0.694 ± 0.115 | 0.247 ± 0.994 |
| L.S.D. | 0.275 | 0.202 |
Tab. 2. Shows the effect of polycystic ovary syndrome and treatment with metformin and asparagine nanocomposite before and after loading it with metformin on the levels of immune parameters (IL-10) and (IL-17) in the serum of female albino rats.
The results from Tab. 2. show varying levels of immune parameters in the studied groups. There is a significant increase (P<0.05) in the level of IL-17 in the PCOS induction group (G2) compared to the control group (G1). Additionally, there is a significant increase (P<0.05) in the level of IL-17 in the group treated with Meto (G3) compared to the control group (G1). The results also demonstrate a significant increase (P<0.05) in the level of IL-17 in the group treated with Asp.NPs (G4) compared to the control group (G1), as well as in the group treated with Asp.NPs./Meto (G5) compared to the control group (G1). Furthermore, the results show a significant decrease (P<0.05) in the level of IL-17 in the group treated with Meto (G3) compared to the PCOS induction group (G2), as well as in the group treated with Asp.NPs (G4) and Asp.NPs./Meto (G5) compared to the PCOS induction group (G2).
The results of the current study revealed that the levels of interleukin-10 (IL-10) were elevated above normal in the PCOS group. This increase is attributed to heightened inflammation in the body, which stimulates the main anti-inflammatory white blood cells. IL-10 functions to suppress the activities of monocytes, macrophages, and pro-inflammatory cytokines like IL-17, IL-10, TNF-ɑ, neutrophils, and T cells. Furthermore, interleukin-10 is linked to obesity and works to counteract pro-inflammatory cytokines that contribute to insulin resistance in women with polycystic ovary syndrome. IL-10 serves as the primary anti-inflammatory cytokine regulating the immune system. According to Al-Hashem [53], treatment with Meto led to a decrease in the level of interleukin-10. This is attributed to the drug's effectiveness in regulating sugar, insulin, and cholesterol levels, which in turn reduces obesity-related inflammation. Additionally, the drug is effective against diabetes and inflammation. As a result, it works to reduce the level of interleukin-10, which acts as an anti-inflammatory and helps alleviate symptoms of polycystic ovary syndrome [54]. Furthermore, the anti-inflammatory effects of fresh asparagus stem extract used in the green nanocomposite preparation can reduce the production of IL-17, IL-6, and TNF-ɑ, while causing an increase in IL-10 in the blood [55]. The therapeutic potential of nanocomposites has led to their widespread use in recent years. When the aqueous extract of fresh asparagus stems is used to prepare green nanocomposites, it increases the effectiveness of asparagine acid present in the extract against inflammation. This results in the production of effective doses of IL-10, which prevents the synthesis of pro-inflammatory cytokines [56]. In various studies, the effect of metformin on body mass index and insulin sensitivity has been observed. It is suggested that there may be a synergistic relationship between the chemical compounds in metformin, which can reduce insulin and lipid levels in the body, enhance glucose absorption, and consequently reduce body mass [57]. We observed a significant increase in the effectiveness of metformin when it was loaded onto the green nanocomposite. This led to positive therapeutic outcomes by reducing the levels of anti-inflammatory interleukins compared to the stimulated group. The enhanced effectiveness is attributed to the green nanosilver compounds, which target inflammatory areas in the body. This combined treatment approach not only reduces obesity-related inflammation but also lowers oxidative stress, thereby reducing anti-inflammatory agents in the body [55,58].
The findings of the recent study revealed that the level of interleukin-17 (IL-17) was elevated above normal in the PCOS group. This increase is attributed to heightened levels of oxidative stress, insulin resistance, ovarian dysfunction, inflammation, obesity, and other characteristics associated with polycystic ovary syndrome. The study also observed the presence of subsets of inflammatory T helper 17 (Th17) cells that produce both IL-17A and IL-17F, and these cells depend on signals generated by high levels of both IL-6 and TNF-ɑ [59]. Stimulation of TGF-β cells with IL-6 leads to increased expression of IL-21 and activation of signal transducer and activator of transcription-3. This activation then leads to the activation of Th17 subsets and drives the proinflammatory IL-17 response [60,61]. Similarly, TNF-ɑ may also cooperate with IL-23 to promote IL-17A expression [62]. Additionally, TNF-ɑ indirectly promotes IL-17A by inducing IL-6 expression [59,63]. Hence, higher levels of IL-6 and TNF-α in females with PCOS may influence the expression of IL-17 cytokines, which could contribute to the development of the disease. Additionally, proinflammatory members of the IL-17 family might also add to the complexity of the inflammatory response in PCOS by working together with TNF-α to stimulate IL-6 expression. IL-17 cytokines have been found to play a significant role in PCOS-related complications such as joint stiffness, a major contributor to cardiovascular disease [64]. Additionally, they have synergistic effects with TNF-α on oxidative stress [59]. Metformin reduces insulin resistance, lipid profile, and cholesterol levels, while enhancing glucose absorption. This helps regulate these factors and reduce inflammation in the body, which has increased due to obesity and oxidative stress [65]. Asparagine nanocomposite Asp.NPs have shown anti-inflammatory activity by reducing the release of pro-inflammatory cytokines (TNF-alpha, IL-6, IL-1 beta, and IL-17). Anti-inflammatory cytokines are regulatory molecules that inhibit the immune response [66]. Nanoparticles can be designed to directly target immune cells and suppress their activity or avoid immune recognition [67]. Aspartate nanoparticles (Asp.NPs) are important in inhibiting inflammatory promoters such as cytokines and proinflammatory enzymes. Many metal and metal oxide nanocomposites, such as silver, have been reported to possess anti-inflammatory properties when compared to their bulk counterparts. Utilizing green silver nanocomposites as an anti-cytokine approach is an interesting strategy to reduce inflammation, as it blocks the interaction between cytokines and their receptors. Polycystic ovary syndrome (PCOS) is a pro-inflammatory condition, and Asp.NPs has a beneficial effect in reducing inflammatory cytokines [68]. The main advantage of nano-drug carriers, such as the metformin-loaded asparagus stem nanocomposite, is to improve the targeted delivery of drugs to the intended site of action. This delivery process is referred to as drug targeting and can be achieved through active or passive targeting methods. By encapsulating biologically active molecules in nanocarriers, their bioavailability can be increased, leading to enhanced stability. Utilizing nanocarriers in drug delivery enhances the quality of drugs, thereby reducing side effects and the required drug dosage. Consequently, this drug, when administered after suppository, has a greater impact on reducing inflammation-causing elements (IL-17 and IL-6) [69,70].
This study demonstrates that the green nanocomposite was successfully prepared from fresh asparagus stems based on the results obtained from AFM and FTIR examination. The study also showed that the loading of metformin on the asparagus nanocomposite was successful, as confirmed by AFM and FTIR examination results. Additionally, it was concluded that the effectiveness of the asparagus nanocomposite, both before and after loading with metformin, in treating polycystic ovary syndrome was high compared to the free metformin drug, and it achieved results in a shorter period of time. Consequently, this bio-prepared nanocomposite reduced side effects, treatment cost, and recovery time.
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.