Research Article - (2023) Volume 0, Issue 0
Received: 02-May-2023, Manuscript No. gpmp-24-129491; Editor assigned: 04-May-2023, Pre QC No. P-129491; Reviewed: 15-May-2023, QC No. Q-129491; Revised: 22-May-2023, Manuscript No. R-129491; Published: 01-Jun-2023
Background: Cell-free DNA (cfDNA) has the potential to provide non-invasive diagnostic assays and practical biomarkers for predicting the outcome of assisted reproductive technology (ART) procedures. Aim of the study: To investigate the association between cf-DNA concentration in individual follicular fluid and oocyte maturity and embryo quality in patients undergoing Intra Cytoplasmic Sperm injection (ICSI).
Patients and methods: This prospective observational clinical study was done at Ain Shams University, A maternity hospital (IVF Unit), and a private ICSI center from May 2019 to June 2020. Our study used the ALU-QPCR technique to accurately measure the cell-free DNA (cfDNA) level in 35 follicular fluid samples from 15 patients undergoing ICSI.
Results: Our results displayed the range of cfDNA from 0.24 to 3.89 (ng/ml), with a mean of 2.06 ± 0.73. The DNA integrity (Q247/Q115) ranged from 0.09 to 0.79, with a mean of 0.44 ± 0.18. Our findings reveal a statistically significant association between the levels of cell-free DNA and patient oocytes to yield an Embryo, top grade Embryos, Fragmentation rate of the embryo, and number of blastomeres (P-values; p=0.037, p<0.001, p<0.001, and p=0.019, respectively ). A statistically negative correlation between cfDNA (ng/ml) and follicle size in mm (p-value < 0.05). However, no significant correlation was observed for the other parameters (basic hormonal profile, AFC number of oocytes, and the number of fertilized oocytes), with a p-value > 0.05. The study found that the levels of cfDNA and 17β-estradiol had a non-significant negative correlation (p-value > 0.05). Our study found that the level of cfDNA in follicular fluid is strongly associated with the quality of embryos on Day 3.
Conclusion: High levels of cfDNA in follicular fluid may indicate poor follicular milieu. Measuring cfDNA can evaluate oocyte and embryo competence. It can be a non-invasive test to assess embryo quality along with morphological criteria.
Cell-free DNA; Oocyte’s; Embryo; Blastomeres
Mandel and Metais discovered cell-free DNAs (cfDNAs) in human serum [1]. DNA fragments are released via different mechanisms, including apoptosis, necrosis, and active release [2]. The length of cfDNAs ranges from 40 to 200 base pairs (bp) on average, but there are also fragments larger than 30 kb that have been detected [3]. These fragments of DNA can be found both in free-circulating form and within extracellular vesicles like exosomes, apoptotic bodies, and microvesicles [4].
Cell-free DNA (cfDNA) has the potential to provide non-invasive diagnostic assays and practical biomarkers for predicting the outcome of assisted reproductive technology (ART) procedures [5]. Researchers have studied the cfDNA profile within its main sources in reproductive medicine, including seminal plasma (SP), follicular fluid (FF), serum, spent culture medium (SCM), and blastocoel fluid (BF) [6,7].
FF (follicular fluid) in ART procedures is considered biological waste material, but it provides an additional environment for cfDNA (cell-free DNA) in women. Studies have investigated the potential of FF cfDNA markers in female reproduction. Assessments on pooled FFs of patients have revealed that more oocytes were related to FFs with lower levels of FF cfDNA [8].
Thus, since most of the studies admitted the significant adverse effect of high levels of FF cfDNA, and only one study considered a non-significant beneficial impact on oocyte developmental potential, a tenable conclusion to draw is that FF cfDNA level has a normal range which has not been discovered yet. Its presence up to a specific level reflects. The oocyte’s successful selection and maturation, while abundant levels impair its developmental potential. Therefore, additional investigations are needed to define a normal cutoff point that will bridge the current gaps [5]. However, research on using FF cfDNA as a diagnostic biomarker for female ART is limited. Establishing a gold standard method is necessary to avoid variations in results before utilizing cfDNA as a biomarker in a clinical setting.
To investigate the association between cf-DNA concentration in individual follicular fluid and oocyte maturity and embryo quality in patients undergoing Intra Cytoplasmic Sperm injection (ICSI).
This prospective observational clinical study was done at Ain Shams University maternity hospital (IVF Unit), and a private ICSI center from May 2019 to June 2020.
The study included 15 patients recruited for ICSI. The study gained ethical committee approval from the institution review board.
Study Outcomes
The primary outcome:
The association between individual mono follicular fluid cf-DNA concentration with oocyte and embryo quality in ICSI.
The secondary outcomes:
Evaluation of the ability of monofollicular cell-free DNA (cfDNA) level to be used as a novel non invasive biomarker to predict different clinical outcomes of ICSI cycles.
Sample size justification: A statistical software called Stats Direct version 2.8 for MS Windows, developed by Stats Direct Ltd. in Cheshire, UK, was used to calculate the required sample size. The aim was to detect the correlation between the concentration of cf-DNA in each follicular fluid and the quality of oocyte maturity and embryo development, according to the study by Scalici et al. [9]. The minimum sample size required to reject the null hypothesis with 80% power (β=0.2) and 95% significance level (α = 0.05) was calculated to be 28 follicular fluid samples. To account for potential dropouts, the sample size was increased by 25%, resulting in 35 follicular fluid samples. These samples will be collected from 15 female patients undergoing IVF/ICSI.
The present study is defined by specific inclusion criteria applied to patient selection. Patients between the ages of 18 and 35, diagnosed with tubal factor of infertility with tubal infertility (without hydrosalpinx), male infertility (excluding azoospermia or low fertility rate), or other factors (including unexplained infertility), normal uterine and cervical morphology, and medical health were included.
The study also employed specific exclusion criteria to ensure the validity and integrity of the data obtained. Patients who were above 35 years of age, women diagnosed with endometriosis, patients with severe male factor (azoospermia requiring testicular biopsy or with a previous low fertilization rate), patients with hydrosalpinx, patients whose follicle-stimulating hormone (FSH) levels were greater than 12 MIU, poor endometrium, and poor ovarian response (POR) were excluded. POR was defined according to the Bologna consensus criteria based on two of three factors: maternal age ≥ 40 years, previous POR, or an abnormal ovarian reserve test (i.e., antral follicular count (AFC) < 5-7 follicles, or anti-müllerian hormone (AMH) < 0.5-1.1 ng/mL). Patients complicated by ovarian hyperstimulation syndrome (OHSS) and those who refused to consent to the use of their data in the study were also excluded. These rigorous criteria ensured that the study participants represented the intended population and minimized potential sources of confounding or bias. All participants in the study were provided with a detailed explanation of the purpose and procedures involved in the research prior to their recruitment. After ensuring that the participants understood the study's objectives, written consent was obtained from each of them. This consent was obtained in a fully informed manner, with all the relevant details about the study being disclosed to the participants. Only after they had provided their written consent did they become part of the study.
Each patient was subjected to treatment via a long agonist stimulation protocol that was initiated on cycle day 21 using triptoreline (Decapeptyl Ferring Pharmaceuticals, Germany) at a dosage of 0.05mg/day S.C. An individualized dose of human menopausal gonadotropin 300-450 IU IM daily (HMG 75 IU, Merional, IBSA) was administered to each patient from days 2 to 3 of the subsequent cycle, following confirmation of down-regulation, which was preceded by an examination of E2 levels. The dose was tailored to each patient based on the diameter of the follicles detected during follow-up vaginal ultrasound. Monitoring was carried out every other day until day 14, with daily monitoring when deemed necessary. When three or more follicles with diameters greater than 16 mm were detected, patients were given Human chorionic gonadotropin (HCG 10,000IU, IM) via intramuscular injection as a triggering hormone at 8 pm on the same day. Oocytes were retrieved 36 hours later.
After the oocytes were retrieved, the granular cells and corona radiata of the cumulus oophorus were removed. The maturity of the oocytes was then evaluated and they were subjected to ICSI treatment. Following fertilization, the zygote was incubated for 18 hours in an IVF nutrient solution at a temperature of 37°C with a 5% CO2 atmosphere. The fertilization status was observed at 24 hours, and the nutrient solution was renewed.
In the process of evaluating Oocyte Corona Cumulus Complexes (OCCC), a stereomicroscope was utilized to assess their maturation. Each OCCC was assigned a grade from zero to four based on specific criteria.
The criteria used to grade the OCCC were as follows: oocytes with a large nucleus in their cytoplasm and a dense corona cumulus layer were deemed immature and assigned a grade of "0". Oocytes lacking a cytoplasmic germinal vesicle and polar body and possessing a corona layer smaller than the total size of five oocytes were assigned a grade of "1st degree". Oocytes with radially placed crowded coronal cells, cumulus cells spreading to a relatively wider area than the second group, containing a polar body, and easily removed from the cell group when manipulated, were assigned a grade of "2nd degree". Oocytes that lost their tight adherence to the coronal cells and had cumulus cells quite scattered but still cellular were assigned a grade of "3rd degree". Oocytes with pale cytoplasm and cumulus cells that lost their cellular image and became gelatinous structures in their vicinity were assigned a grade of "4th degree" mature in the classification (M2 and Late M2). It is worth noting that M2 oocytes have clear cytoplasm, normal cell size, normal zona pellucida, and a non-fragmented polar body. Late M2 oocytes are post-mature oocytes that have dark cytoplasm with cytoplasmic granulations, abnormalities in the zona pellucida, and fragmented polar bodies. M2 oocytes are considered to be superior to late M2 oocytes.
The following is a summary of the procedures involved in IVF, specifically semen collection, sperm preparation, and embryo culture. Semen collection and sperm preparation involves the careful extraction of semen and the processing of the sample to yield viable sperm for use in fertilization.
• Embryo culture begins with the injection of oocytes and proceeds with a check for fertilization and an embryo morphology assessment. Fertilization is ascertained by examining morphological indications, including the appearance of two pronuclei and the extrusion of the second polar body. The embryo's morphology is assessed at or near the transfer point, with early embryos analyzed for the total number of blastomeres, the presence of blastomere regularity, and the extent of fragmentation.
• These procedures are critical in the IVF process and require careful attention to detail by all involved parties to ensure optimal outcomes.
Multinucleation compaction of blastomeres as:
Embryo Grading System The following is a list of ratings and their respective descriptions for embryo grading in the context of assisted reproductive technologies:
Grade 1: Good - Fragmentation of less than 10% - 6-8 blastomeres - Stage-specific cell size - No multinucleation.
Grade 2: Fair - Fragmentation between 10% and 25% - 6-8 blastomeres - Stage-specific cell size for the majority of cells - No evidence of multinucleation .
Grade 3: Poor - Severe fragmentation, more than 25% - Cell size not stage-specific - Presence of multinucleation.
Grade 4: Poor - Severe fragmentation, more than 40% - Cell size not stage-specific - Irregular blastomeres in the context of assisted reproductive technologies, embryo grading is an essential factor in determining the viability of embryos for procedures such as in vitro fertilization. The above-described criteria provide a standardized method for assessing embryos and determining their suitability in these procedures [9].
The three most viable embryos were transferred to the uterus on the third day, between 66-74 hours after insemination. If possible, embryo transfers were done during the second or third day after oocyte collection, when the embryos were in at least the two-cell stage. It is important to note that we only included patients who underwent fresh embryo transfer.
VII. Follicular fluid processing and Cell-free DNA extraction and quantification : During oocyte retrieval, follicular fluids were aspirated individually without flushing for each patient. Follicle aspirates that were unclear, such as those contaminated with blood, were discarded. After collecting the oocytes, the follicular fluid was centrifuged at 3000g for 15 minutes, and the supernatant was filtered with 0.22 μm filters to eliminate cell debris, including granulosa cells, erythrocytes, and leukocytes. The volume of each follicular fluid sample was measured, and the diameter of the corresponding follicle was calculated based on the follicular fluid volume. The samples were frozen at -80°C until cfDNA quantification. Follicular fluid samples were prepared as described above for cell-free DNA extraction and quantification. Briefly, 20 µl of each follicular fluid sample was mixed with 20 µl of a buffer containing 25 ml/l Tween 20, 50 mmol/l Tris, and one mmol/l EDTA. The sample was then digested with 16 µg of proteinase K (PK) (Qiagen) at 50 °C for 20 minutes, followed by heat inactivation and insolubilization at 95°C for 5 minutes. The samples were centrifuged at 10000g for 5 minutes, and supernatants were collected and stored at -80°C until cfDNA quantification. CfDNA was quantified by qPCR for human ALU repeats using two primer sets that generate a 115-bp amplicon (ALU115 primers) and a 247-bp amplicon (ALU247 primers), respectively. For each ALU-qPCR, 1 µl of each PK-digested follicular fluid sample was added to a reaction mixture (final volume: 10 µl) containing 0.25 µM of forward and reverse primers (ALU115 or ALU247) and 5 µl of 2X LightCycler®480 SYBR Green I master mix (Roche Applied Science, Germany). Follicular fluid cfDNA concentrations were calculated based on a standard curve prepared with successive dilutions of genomic DNA. A negative control (without a template) was added to each qPCR plate. All measures were performed in quadruplicate [9].
The following hormonal assays were conducted: FSH, estradiol, and LH. These were measured using Chemiluminescent Microparticle Immunoassay kits (Architect Abbott Lab, IL, USA). The concentration of E2 in follicular fluid samples was determined by Immunochemiluminescence using Cobas e411 kits from Roche Diagnostics.
Progesterone (Cyclogest 400 mg TDS vaginal sup.) administration was used in all cases starting on the day of oocyte retrieval.
Pregnancy test was done at the 14th day post transfer in case of day 3 ET.
We performed ALU 115-qPCR to measure the total cfDNA present in 35 samples of follicular fluid collected from individual follicles. The concentration of cfDNA (ng/ml) was measured in the range of 0.24 to 3.89, with an average of 2.06 ± 0.73. Furthermore, we also assessed the DNA integrity (Q247/Q115) in these samples. We found that the DNA integrity ranged from 0.09 to 0.79, with a mean of 0.44 ± 0.18.
Tab. 1. provides a comprehensive overview of the different demographic data, including age, BMI, and duration of infertility, alongside relevant information on infertility, such as duration and etiology. Additionally, the table presents hormonal profile results, including FSH, LH, estradiol, AMH, and 17β-estradiol, as well as the total dose of gonadotropins IU.
| Baseline characteristics | Total (n=15) |
|---|---|
| Age "years" | |
| <30 years | 8 (53.3%) |
| ≥30 years | 7 (46.7%) |
| Range, Mean ± SD | 20-35 (28.33 ± 4.39) |
| BMI (kg/m2) | |
| Normal weight | 3 (20.0%) |
| Overweight | 6 (40.0%) |
| Obese | 6 (40.0%) |
| Range, Mean ± SD | 19-32 (27.27 ± 3.99) |
| Infertility duration (years) | |
| Range, Mean ± SD | 2-9 (7.00 ± 2.30) |
| Infertility etiology | |
| Female factor | 3 (20.0%) |
| Male factor | 3 (20.0%) |
| Mixed infertility | 2 (13.3%) |
| Ovulatory dysfunction | 2 (13.3%) |
| Tubal alterations | 2 (13.3%) |
| Unexplained infertility | 2 (13.3%) |
| Ovarian disorders | 1 (6.7%) |
| Hormonal profile | |
| FSH (IU/l) Range, Mean ± SD | 4.8-10 (7.16 ± 1.64) |
| LH (IU/l) Range, Mean ± SD | 1.5-8.1 (3.15 ± 1.97) |
| E2 (pg/ml) Range, Mean ± SD | 20-56 (35.80 ± 12.59) |
| AMH (ng/ml) Range, Mean ± SD | 1-3.9 (1.90 ± 0.89) |
| 17β-estradiol (ng/ml) Range, Mean ± SD | 1220-4548 (2270.07 ± 874.10) |
| Total dose of gonadotropins IU | 3150-5025 (4048.33 ± 643.54) |
Tab. 1. Baseline characteristics distribution among study group.
Tab. 2. provides a comprehensive overview of various factors related to the study group. It includes data on the antral follicle count, which measures ovarian reserve, and the number of oocytes retrieved during the stimulation process. The table also lists the number of days for which the participants were stimulated and the size of the follicles at the time of retrieval, as these factors can impact the procedure's success.
| Variables | Total (n=15) |
|---|---|
| Antral follicle count; Range [Mean±SD] | 7-13 [9 ± 2] |
| Number of oocytes; Range [Mean±SD] | 6-16 [8 ± 3] |
| Number of stimulation days; Range [Mean±SD] | 13-17 [16 ± 1] |
| Follicle size (mm) [n=35]; Range [Mean±SD] | 18-25 [21 ± 2] |
| Normal fertilized oocyte; Range [Mean±SD] | 3-14 [5 ± 2] |
| Normality fertilized oocyte (n=35) | |
| No normal fertilized oocyte | 5 (14.3%) |
| Normal fertilized oocyte | 30 (85.7%) |
| Number of embryos; Range [Mean±SD] | 2-3 [2 ± 1] |
| Oocytes maturity (n=35) | |
| MI (immature) | 11 (31.4%) |
| MII (mature) | 24 (68.6%) |
| Number of Embryo (n=35) | |
| No Embryo | 5 (14.3%) |
| Embryo | 30 (85.7%) |
| Embryo quality (n=30) | |
| Grade 1 | 7 (23.3%) |
| Grade 2 | 7 (23.3%) |
| Grade 3 | 6 (20.0%) |
| Grade 4 | 10 (33.3%) |
| Embryo quality (n=30) | |
| Top embryo (Grades 1 and 2) | 14 (46.7%) |
| No top embryo (Grades 3 and 4) | 16 (53.3%) |
| Cleavage (n=30) | |
| No early cleavage | 20 (66.7%) |
| Early cleavage | 10 (33.3%) |
| Fragmentation rate embryo (n=30) | |
| Fragmentation ≤25% | 14 (46.7%) |
| Fragmentation >25% | 16 (53.3%) |
| No. of Blastomers (n=30) | |
| <6 cells | 12 (40.0%) |
| 6-8 cells | 7 (23.3%) |
| >8cells | 11 (36.7%) |
Tab. 2. Outcomes of IVF/ICSI.
Furthermore, the table provides information on the number of embryos obtained, the number of immature oocytes, and the cleavage rate of the embryos. The fragmentation rate of the embryos is also listed, as it is an essential indicator of the quality of the embryos. Finally, the number of blastomers distribution among the study group is included, which provides insight into the early growth and development of the embryos.
The findings presented in Tab. 3. demonstrate a statistically significant relationship between the levels of cell-free DNA and several key factors that contribute to successful embryo development. Specifically, the results indicate a strong correlation between cell-free DNA levels and the following characteristics: patient oocyte yielded an Embryo, Fragmentation rate embryo, and No of blastomeres.
| Variables | cfDNA (ng/ml) | b ± SE | p-value |
|---|---|---|---|
| Mean ± SD [95% C.I.] | |||
| Immature oocytes | |||
| Immature oocytes (MI) | 2.34 ± 1.23 [1.76-2.93] | 0.65 ± 0.80 | 0.344 |
| Mature oocytes (MII) | 1.93 ± 0.99 [1.45-2.41] | ||
| Normality fertilized oocyte | |||
| No normal fertilized oocyte | 2.36 ± 0.68 [1.77-2.95] | 0.71 ± 0.63 | 0.247 |
| Normal fertilized oocyte | 1.92 ± 0.72 [1.44-2.40] | ||
| Embryos | |||
| No Embryo | 3.16 ± 0.68 [2.37-3.95] | 0.20 ± 0.36 | 0.037* |
| Embryo | 1.88 ± 1.02 [1.41-2.35] | ||
| Cleavage | |||
| No early cleavage | 1.87 ± 0.71 [1.40-2.34] | -0.82 ± 0.70 | 0.651 |
| Early cleavage | 1.69 ± 0.63 [1.27-2.11] | ||
| Embryo Top | |||
| Top embryo (Grades 1 and 2) | 1.17 ± 0.60 [0.88-1.46] | 2.34 ± 0.67 | <0.001** |
| No top embryo (Grades 3 and 4) | 2.50 ± 0.90 [1.87-3.12] | ||
| Fragmentation rate embryo | |||
| ≤25% | 1.17 ± 0.60 [0.88-1.46] | 2.80 ± 0.76 | <0.001** |
| >25% | 2.50 ± 0.90 [1.87-3.12] | ||
| No. of blastomeres | |||
| <6 cells | 2.43 ± 0.90 [1.83-3.04] | -0.48 ± 0.19 | 0.019* |
| 6-8 cells | 1.82 ± 1.30 [1.37-2.28] | ||
| >8cells | 1.30 ± 0.60 [0.98-1.63] | ||
Tab. 3. Association between cell-free DNA levels in follicular fluid and IVF outcomes.
The p-values associated with these factors are noteworthy, with values of p=0.037, p<0.001, p<0.001, and p=0.019, respectively. These findings suggest that variations in cell-free DNA levels may significantly impact the development of embryos and, as a result, could have implications for fertility treatment and research.
According to the results presented in Tab. 4. a negative correlation exists between cfDNA (ng/ml) and follicle size (mm), which has been statistically proven with a p-value of less than 0.05. However, no significant correlation was observed for the other parameters, which have been tested with a p-value of more than 0.05.
| Parameters | cfDNA (ng/ml) | |
|---|---|---|
| r-value | p-value | |
| Age (years) | -0.267 | 0.336 |
| BMI (kg/m2) | 0.030 | 0.914 |
| Infertility duration (years) | -0.272 | 0.327 |
| Cycle number | -0.416 | 0.123 |
| FSH (IU/l) | 0.015 | 0.956 |
| LH (IU/l) | -0.019 | 0.945 |
| E2 (pg/ml) | -0.060 | 0.831 |
| AMH (ng/ml) | 0.025 | 0.931 |
| Total dose of gonadotropins | 0.066 | 0.816 |
| Antral follicle count | -0.005 | 0.987 |
| Number of oocytes | -0.085 | 0.763 |
| Number of days stimulation | -0.086 | 0.761 |
| Follicle size (mm) | -0.631 | <0.001** |
| Normal fertilized oocyte | -0.084 | 0.767 |
| Number of embryo | 0.360 | 0.188 |
Tab. 4. Correlation between cfDNA (ng/ml) with different parameters.
The study found that the levels of cfDNA (cell-free DNA) and 17β-estradiol (a form of estrogen) had a non-significant negative correlation, meaning that changes in one did not significantly affect the other. However, the study did uncover a significant negative correlation between DNA integrity (as measured by the Q247/Q115 ratio) and 17β-estradiol levels, indicating that estrogen changes may impact DNA integrity. These findings are illustrated in Tab. 5. as well as in Fig. 1. and 2.
| Variables | 17β-estradiol (ng/ml) | |
|---|---|---|
| r-value | p-value | |
| cfDNA (ng/ml) | -0.060 | 0.831 |
| DNA integrity (Q247/Q115) | -0.382 | 0.004* |
Tab. 5. Correlation between DNA integrity (Q247/Q115) and cfDNA with 17β-estradiol (ng/ml), using Pearson’s correlation coefficient (r).
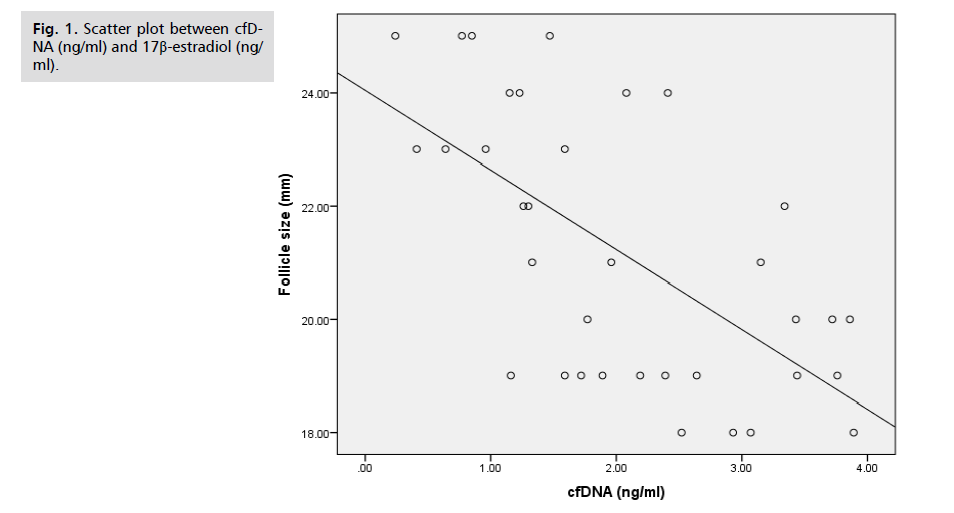
Fig 1. Scatter plot between cfDNA (ng/ml) and 17β-estradiol (ng/ml).

Fig 2. Scatter plot between DNA integrity (Q247/Q115) and 17β-estradiol (ng/ml).
In 2004, Hart and colleagues submitted the inaugural report on the use of cfDNA in the female reproductive field. The primary objective of their study was to assess the value of serum cfDNA as a pregnancy marker. To accomplish this, they analyzed the serum samples of women one week after embryo transfer. According to the report, there was no correlation between the serum cfDNA concentration or fragment size distribution and pregnancy achievement [10].
Numerous studies have explored the potential of serum cfDNA biomarkers in female reproduction. Research indicates that heightened levels of serum cfDNA may be linked to decreased pregnancy rates [11,12].
Our results and their interpretation
Our study used the ALU-QPCR technique to accurately measure the cell-free DNA (cfDNA) level in follicular fluid samples. We employed two different types of primers in our real-time quantitative PCR to achieve this. The first set of primers, plate number 1, allowed us to measure the total amount of cfDNA in the sample. The second set of primers, plate number 2, only amplified large cfDNA fragments derived from cellular necrosis, not apoptosis. Using the Q247/Q115 ratio of the cfDNA integrity index, we determined the amount of cfDNA generated by cellular necrosis, providing invaluable insight into the underlying cellular mechanisms at work.
Our results displayed the range of cfDNA (ng/ml) from 0.24 to 3.89, with a mean of 2.06 ± 0.73. The DNA integrity (Q247/Q115) ranged from 0.09 to 0.79, with a mean of 0.44 ± 0.18.
Our findings reveal a statistically meaningful association between the levels of cell-free DNA and various crucial factors that play a role in the development of embryos. To be precise, the results suggest a robust correlation between the levels of cell-free DNA and the following characteristics: patient oocytes to yield an Embryo, top grade Embryos, Fragmentation rate of the embryo, and number of blastomeres.
The p-values associated with these factors are noteworthy, with values of p=0.037, p<0.001, p<0.001, and p=0.019, respectively. These findings suggest that variations in cell-free DNA levels may significantly impact the development of embryos and, as a result, could have implications for fertility treatment and research.
Our results show a noteworthy negative correlation between cfDNA (ng/ml) and follicle size (mm), statistically proven with a p-value of less than 0.05. However, no significant correlation was observed for the other parameters (basic hormonal profile, AFC number of oocytes, and the number of fertilized oocytes), which have been tested with a p-value of more than 0.05.
Our study suggests a strong correlation between the E2 level within the follicle and the prevalence of necrotic events. We found that a reduction of E2 within the follicle was linked to an increase in necrotic events, which was, in turn, associated with a higher level of cell-free DNA (cfDNA) integrity index (Q247/Q115 ratio). This negative correlation was statistically significant and suggests that the level of E2 within the follicle may be a crucial determinant of the extent of damage and cell death that occurs within it.
Moreover, our findings indicate that the amount of cfDNA present in mono follicular fluid samples is significantly and negatively correlated with follicle size. This suggests that the level of cfDNA may be an indicator of the functional and maturity state of each follicle. This association between cfDNA and follicle size highlights the importance of monitoring cfDNA levels as a potential biomarker for predicting the reproductive potential of an individual.
In addition, our study also revealed a strong and significant association between the level of cfDNA in follicular fluid samples and the morphological quality of embryos on Day 3. Specifically, we observed that the follicular fluid of oocytes that produced grade 1 and 2 embryos with low fragmentation rates (≤25%) had significantly higher cfDNA concentration when compared to the follicular fluid of oocytes that developed grade 3 and 4 embryos with high fragmentation rates (>25%). These findings suggest that the level of cfDNA in the follicular fluid could serve as a valuable prognostic marker for predicting embryo quality and developmental potential.
Our findings revealed a correlation between the rate at which embryos divide and the amount of cell-free DNA (cfDNA) present in the follicular fluid. We found that follicles containing high levels of cfDNA in the fluid tend to produce embryos that divide slowly and have a high fragmentation rate. Such embryos often have only six blastomeres on Day 3, indicating slower development. This information can help us understand the factors that affect embryo development and may have implications for fertility treatments.
Comparison of our results to different studies
Many studies have been conducted to investigate the potential of the FF cfDNA marker and its application in female reproduction. These studies have examined FF cfDNA in individual follicles or pooled FFs of each patient [5]. Assessments of follicular fluids from patients undergoing controlled ovarian stimulation (COS) revealed that more retrieved oocytes were associated with follicular fluids with lower levels of cell-free DNA. [8,13].
Recent studies have investigated the association between follicular fluid (FF) cell-free DNA (cfDNA) levels and oocyte maturity stage, revealing no significant correlation between the two. In other words, the amount of cfDNA present in individual follicles or pooled FFs does not appear to indicate whether an oocyte is at the metaphase II (MII) or metaphase I (MI) stage of maturity. [9,14]. The study's findings indicate that the level of FF cfDNA does not significantly change during the different stages of follicle development. The biomarker may be more sensitive to pathological conditions than normal physiological processes. If this hypothesis is true, it could add further weight to the potential use of FF cfDNA as a reliable biomarker in female reproductive medicine. The study provides valuable insights into the application and interpretation of FF cfDNA levels in clinical settings, which could aid in diagnosing and treating various reproductive disorders [5].
Despite various attempts to investigate the correlation between FF cfDNA and ART outcome, no significant interrelation was found with fertilization success rate. However, the research discovered a noteworthy correlation between FF cfDNA and embryo quality. This suggests that FF cfDNA could be a potential biomarker to predict embryo quality during ART treatments [9,14].
In their initial reports, Dimopoulou and her colleagues conducted a study investigating the relationship between the concentration of FF cfDNA in pooled FFs and embryo quality. The study analyzed the data in detail and found no significant correlation. This suggests that the concentration of FF cfDNA may not be a reliable indicator of embryo quality [8]. The study conducted by Scalici et al. revealed a significant association between the quality of day three embryos and the level of cell-free DNA (cfDNA) present in the follicles related to the oocyte. The study found that oocytes related to follicles with lower levels of cfDNA had a greater chance of developing into high-quality embryos. In comparison, oocytes related to follicles with higher levels of cfDNA were more likely to develop into low-quality embryos. The authors recommended measuring cfDNA levels in follicular fluid as a biomarker for assessing the quality of day three embryos. This approach can be a valuable tool for embryologists to select the best embryos for transfer and ultimately improve the success rates of in vitro fertilization (IVF) treatment [9].
Further studies have been conducted on cell-free DNA (cfDNA) in follicular fluid (FF) samples, both individually and as pools. These studies have confirmed a negative correlation between the levels of cfDNA in FF and the quality of day 3 embryos. This suggests that higher levels of cfDNA may be detrimental to embryo development and may indicate poor embryo quality [13-18]. Liu and colleagues conducted a study that investigated the relationship between FF cf-nDNA and FF cf-mtDNA levels and the quality of day three embryos. Contrary to previous agreements, their study found no correlation between these factors and day 3 embryo quality. However, the study uncovered a relationship between blastocyst developmental competence and FF cf-nDNA and FF cf-mtDNA levels. S This finding suggests that these factors may play a more significant role in later-stage embryo development than early development [15].
This study aimed to investigate the relationship between the levels of cell-free mitochondrial DNA (cf-mtDNA) and cell-free nuclear DNA (cf-nDNA) in follicular fluid (FF) and the developmental potential of oocytes. The results showed that the follicles with oocytes that developed into viable blastocysts had lower levels of FF cf-mtDNA but higher levels of FF cf-nDNA compared to those that did not reach the blastocyst stage. The difference in FF cf-mtDNA was found to be significant, indicating that it could be a promising biomarker for predicting blastocyst developmental potential. [15].
The available data shows that high levels of FF cfDNA have a significant negative impact on the oocyte's developmental potential in most studies. However, a single study has suggested that it could have a non-significant positive effect. It is therefore reasonable to conclude that there may be a normal range of FF cfDNA levels, which has not yet been discovered, that indicates whether an oocyte has been successfully selected and matured. Conversely, excessive levels of FF cfDNA can impair its developmental potential. As a result, further research is needed to determine the normal cutoff point that can fill in the gaps in the current understanding of FF cfDNA levels and their impact on oocyte development [5].
Through continuous efforts to find FF cfDNA biomarkers in the ART field, a significant association with pregnancy outcomes has been discovered. Except one study [8], all accepted a negative correlation between FF cfDNA levels and pregnancy, making it a highly sensitive and specific biomarker for predicting pregnancy achievement. [9,16-19].
However, there are some controversies regarding the exact type of correlations (positive/negative) between FF cfDNA level and embryo quality that, as mentioned above, might be due to the lack of the cutoff point definition. On the other side, since the impact of COS type on FF cfDNA level has been proved by some studies [13,15], it has to be considered that distinct stimulation protocols might require their specific cutoff point.
Strengths and limitations of our study: the strength of our study is its appropriate methodology and potency of our embryologists, while its limitation is the small number of patients, but this was justified by proper sample size justification.
Recommendations for further studies: More research is needed to determine the cutoff point for different situations and to assess the genetic and epigenetic status of FF cfDNA. This will help us better understand FF cfDNA and increase its use in female reproductive medicine. A more extensive study is needed to investigate the relationship between cfDNA level in follicular fluid samples and implantation rate, independently of embryo morphology.
High levels of cfDNA in follicular fluid samples may indicate a poor follicular milieu. Measuring the concentration of cfDNA in follicular fluid can be used to evaluate the developmental competence of oocytes and embryos. Using cfDNA follicular fluid samples as a non-invasive test for assessing embryo quality can be added to the classic morphological criteria.
The study gained ethical committee approval from the National Institute of Laser Enhanced Sciences (N.I.L.E.S) - Cairo University with the number EC Ref No: 018- 010 which runs with declaration of Helsinki.
Data can be shared if required.
No competing interests exist.
No funding disclosures or support to make with regard to this study.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.