Research - (2022) Volume 17, Issue 4
Received: 14-Aug-2022, Manuscript No. gpmp-22-71882; Editor assigned: 16-Aug-2022, Pre QC No. P-71882; Reviewed: 28-Aug-2022, QC No. Q-71882; Revised: 12-Sep-2022, Manuscript No. R-71882; Published: 29-Dec-2022
Background: Preeclampsia is a multisystmic disorder of unknown cause. Endothelial cell damage has recently been suggested to underlie the pathologic change in preeclamptic pregnancy. Thrombomodulin an endothelial cell surface glycoprotein act as a co-factor for thrombin catalyzed activation of protein C. activated protein C inhibits coagulation by inactivation the coagulation factor Va and VIIIa.
Aim of the Work: to assess the changes in thrombomodulin level in women with preeclampsia. Patients and Methods: This prospective case-control study was conducted on 123 women at Ain Shams University Maternity Hospital.
Results: Regarding clinic-pathological features of pre-eclampsia patients and healthy control groups, our study found that there was high significant difference (p≤0.01) between hypertensive and normal patients regarding (hypertension, obesity and history of PET). Our study found that there was high significant difference (p≤0.01) between pre-eclampsia patients and healthy control group regarding serum thrombomodulin protein level and serum thrombomodulin protein increases significantly with mild and severe preeclampsia and HELLP syndrome and considered a good marker for evaluation of hypertensive patients with pregnancy.
Conclusion: Serum thrombomodulin protein level is considered a good marker for evaluation of hypertensive patients with pregnancy.
Uterine leiomyoma; Vitamin D
Preeclampsia is a multisystemic disorder of unknown cause. Endothelial cell damage has recently been suggested to underlie the pathologic change in preeclamptic pregnancy. Thrombomodulin an endothelial cell surface glycoprotein act as a co-factor for thrombin catalyzed activation of protein C. activated protein C inhibit coagulation by inactivation the coagulation factor Va and VIIIa [1].
Pre-eclampsia (PE) is a pregnancy specific, multisystem disorder characterized by reduced organ perfusion secondary to diffuse endothelial injury [2].
The condition complicates about 3-6% of pregnancies worldwide. Despite extensive research, the exact etiology of pre-eclampsia remains elusive [3]. Severe preeclampsia is associated with increased risk of maternal mortality (0.2%) and increased rates of maternal morbidity (5%) such as convulsions, pulmonary edema, acute renal or liver failure, liver hemorrhage, disseminated intravascular coagulopathy (DIC), and stroke. These complications are commonly seen in women with preeclampsia that develops before 28 weeks (early onset PE) gestation and in those with preexisting medical conditions [4].
Serum uric acid levels have been reported to be significantly elevated in subjects with preeclampsia in many studies. Some authors suggest that the degree of elevation correlates with the severity of the maternal syndrome and of fetal morbidity [5].
Lactate Dehydrogenase (LDH) levels were significantly elevated in women with preeclampsia and eclampsia. Higher LDH levels had significant correlation with high blood pressure as well as poor maternal and perinatal outcome [6].
Thrombomodulin (TM); is a cell surface-expressed transmembrane glycoprotein which was originally identified on vascular endothelium. It acts as a natural anticoagulant. Endothelial injury results in TM release [7].
Thrombomodulin is a critical cofactor in the initiation of the protein C (PC) anticoagulant pathway. Plasma levels of thrombomodulin are regulated on a genetic basis, but more important is the dependence on a series of other atherosclerotic risk factors, such as hypertriglyceridemia, stroke, cancer, and diabetes [8]. In normal pregnancy, activation of platelets and release of thromboglobulin and platelet factor 4 are documented. Increased expression of thrombo-modulin is now considered an independent risk factor for vascular disease [9].
To detect that if there is significant relation between the serum level of thrombomodulin and degree of severity of cases of preeclampsia.
This A prospective case-control study was conducted on Department of Obstetrics & Gynecology & Ain shams University maternity hospital.
Study population
Women attending hospital antenatal clinics and labour words of Ain Shams University Maternity hospital.
Inclusion Criteria: We had three groups of pregnant women in the third trimester. The first group is normotensive women. The second group is pregnant women in the third trimester with non-severe preeclampsia (Preeclampsia is defined as sustained Blood pressure ≥ 140/90 in 2 occasions 6 hours apart (not more than 1 week apart) plus Proteinuria ≥ 300mg/24-h urine after 20 weeks of gestation).
The third group is pregnant women in the third trimester of pregnancy with severe preeclampsia (severe preeclampsia is defined as systolic blood pressure more than 160 or diastolic blood pressure more than 110. Also if there is systemic affection as HELLP syndrome).
Exclusion criteria: Pregnant women with history of deep vein thrombosis or known hypercoagulable state for example thrombophilia, pregnant women with chronic hypertension, pregnant women with cardiovascular, autoimmune, renal or hepatic diseases and pregnant women who have diabetes were excluded from the study.
Sample size justification: Sample size was calculated using G* Power versio 3.1.9.2, setting the power (β) at 0.02 and the significance level (a) at 0.02. Data from previous reports [10] indicated that the mean serum thrombomodulin level in control, mild preeclampsia and severe preeclampsia patients was 9.8 ± 4.92, 12.5 ± 6.0 and 21.2 ± 10.84 ng/mL respectively. Calcualtion according to these values produced a minimal total sample size of 117 women. Assuming a drop-out rate of 5%, a total drop-out inflated sample size of 123 women approximately was needed; with 41 women in each study group.
Procedures
All patients were subjected to the following:
Patient's evaluation focusing on detailed history including the onset of the maternal syndrome and detailed clinical examination.
Laboratory investigations included CBC, serum alanine aminotransferase (ALT) and, Aspartate aminotransferase (AST) levels, Serum creatinine, Coagulation profile (prothrombin time [PT], activated partial thromboplastin time [aPTT], and fibrinogen, serum lactate dehydro-genase level (LDH), plasma thrombomodulin (TM) was measured with enzyme Linked Immunosorbent assay (ELISA), Urine: albumin assessment by dipstick, Urinary albumin creatinine ratio (uACr).
Methodology
The subjects consisted of 41 patients with non-severe preeclampsia, 41 normotensive pregnant women in third trimester, and 41pregnant female with sever preeclampsia. Blood samples were collected before any medical intervenion and centrifuged for 20 min at 2,000 rpm. The resulting plasma samples were stored at -70°C until assayed. Preeclampsia defined as a systolic or diastolic blood pressure > 140 or 90 mm Hg, respectively, on two occasions recorded 24 h apart with the presence of proteinuria (>500 mg/day) in a woman who had been normotensive during the first 20 weeks of pregnancy. Plasma TM was assayed by a one-step sandwich enzyme immunoassay using monoclonal antibodies to human TM using kits. This kit is used to assay the Thrombomodulin (TM) in the sample of Human’s serum.
Test principle: This kit uses enzyme-linked immune sorbent assay (ELISA) based on the Biotin double antibody sandwich technology to assay the Human Thrombomodulin (TM). Add Thrombomodulin (TM) to the wells, which are pre-coated with Thrombomodulin (TM) monoclonal antibody and then incubate. After that, add anti TM antibodies labeled with biotin to unite with streptavidin-HRP, which forms immune complex. Remove unbound enzymes after incubation and washing. Add substrate A and B. Then the solution turned blue and changed into yellow with the effect of acid.
To eliminate interassay variation, all 123 samples were assayed in the same run. Data were analyzed statistically using paired Student’s t test. A level of p < 0.05 was accepted as statistically significant.
Statistical analysis: The collected data were revised, coded, tabulated and introduced to a PC using Statistical package for Social Science (SPSS 20.0.1 for windows; SPSS Inc, Chicago, IL, 2001). Quantitative variables are expressed as mean and SD, or Median and Interquartile range (IQR) according to distribution of data. Qualitative variables are expressed as frequencies and percents. Student t test and Mann Whitney test were used to compare a continuous variable between two study groups. Chi square test were used to examine the relationship between Categorical variables. P-value: Level of significance: P>0.05: Non-significant (NS), P<0.05: Significant (S) and P<0.01: Highly significant (HS).
Descriptive analysis of demographic characteristics of pre-eclampsia and control groups are shown in Tab. 1. Descriptive analysis of demographic characteristics of mild and severe pre-eclampsia sub-groups are shown in Tab. 2. Descriptive analysis of clinic-pathological features of pre-eclampsia patients and healthy control groups are shown in Tab. 3. Descriptive analysis of clinic-pathological features of studied groups are shown in Tab. 4. Comparative analysis for serum thrombomodulin protein level between pre-eclampsia patients and healthy control group Mann-Whitney test are shown in Tab. 5. Comparative analysis for serum thrombomodulin protein level between mild and severe pre-eclampsia patients group Mann-Whitney test are shown in Tab. 6. Prognostic potential of serum thrombomodulin protein level in discrimination between mild and severe cases of pre-eclampsia; ROC curve analysis test are shown in Tab. 7.
| Variable | Statistics | Preeclampsia n=82 |
Control n-41 |
|---|---|---|---|
| Age (years) | mean±SD | 31.5±5.0 | 22.7±4.5 |
| Range | 20 - 42 | 17 - 34 | |
| Gestational age (weeks) | mean±SD | 34.17±3.0 | 34.2±3.2 |
| Range | 28 - 39 | 28 - 38 | |
| Gravity (number) | mean±SD | 2.0±1.0 | 2.2±0.8 |
| Range | 1 - 5 | 1 - 3 | |
| Parity (number) | Median (IQR) | 1(2) | 1 (2) |
| Range | 0 - 3 | 0 - 2 | |
| BMI (kg/m2) | mean±SD | 32.0±3.8 | 21.0±5.0 |
| Range | 24 - 38 | 18 - 33 |
Tab. 1. Descriptive analysis of demographic characteristics of pre-eclampsia and control groups.
| Variable | Statistics | Mild Preeclampsia N=41 |
Severe preeclampsia N=41 |
|---|---|---|---|
| Age (years) | mean±SD | 28.4±4.0 | 34.6±4.0 |
| Range | 20 - 39 | 22 - 42 | |
| Gestational age (weeks) | mean±SD | 33.3±3.3 | 36.0±2.6 |
| Range | 28 - 39 | 28 - 38 | |
| Gravity (number) | mean±SD | 2.0±1.0 | 1.98±0.9 |
| Range | 1 - 5 | 1 - 5 | |
| Parity (number) | Median (IQR) | 1(2) | 1 (2) |
| Range | 0 - 3 | 0 - 3 | |
| BMI (kg/m2) | mean±SD | 31.0±4.0 | 32±3.4 |
| Range | 24 - 38 | 24 - 38 |
Tab. 2. Descriptive analysis of demographic characteristics of mild and severe preeclampsia sub-groups.
| Variable | Total | Preeclampsia N=82 |
Control n-41 |
Statistics | P value |
|---|---|---|---|---|---|
| History of CS | |||||
| Negative | 75(61) | 54(66) | 21(28) | U=2.4 | 0.08 (NS) |
| Positive | 48(39) | 28(34) | 20(42) | ||
| Number of CS | |||||
| One CS | 23(48) | 15(53) | 8(35) | U=5.4 | 0.001 (NS) |
| Two CS | 21(44) | 9(33) | 12(57) | ||
| Three CS | 4(8) | 4(14) | 0 | ||
| Hypertension | |||||
| Negative | 49(40) | 16(20) | 33(67) | U=42.4 | 0.001 (HS) |
| Positive | 74(60) | 66(80) | 8(11) | ||
| Blood Pressure | |||||
| Normotensive | 12(10) | 8(10) | 4(33) | F=56.0 | 0.001 (HS) |
| Hypertensive | 66(54) | 54(66) | 12(18) | ||
| Hypotensive | 45(37) | 20(24) | 25(56) | ||
| BMI (Kg/m2) | |||||
| Healthy (18 – 25) | 41(33) | 8(10) | 33(8) | U=61.5 | 0.001 (HS) |
| Obese (>25) | 82(67) | 74(90) | 8(10) | ||
| History of PET | |||||
| Negative | 85(70) | 44(54) | 41(48) | U=27.5 | 0.001 (HS) |
| Positive | 38(30) | 38(46) | 0 | ||
Tab. 3. Descriptive analysis of clinic-pathological features of pre-eclampsia patients and healthy control groups.
| Variable | Total | Mild Preeclampsia N=41 |
Severe preeclampsia N=41 |
Statistics | P value |
|---|---|---|---|---|---|
| History of CS | |||||
| Negative | 54(66) | 27(36) | 27(36) | U=0.0 | 0.6 (NS) |
| Positive | 28(34) | 14(29) | 14(29) | ||
| Number of CS | |||||
| One CS | 15(54) | 7(30) | 8(35) | U=0.18 | 0.9 (NS) |
| Two CS | 9(32) | 5(24) | 4(19) | ||
| Three CS | 4(14) | 2(50) | 2(50) | ||
| Hypertension | |||||
| Negative | 16(20) | 16(33) | 0 | U=19.8 | 0.001 (HS) |
| Positive | 66(80) | 25(34) | 41(55) | ||
| Blood Pressure | |||||
| Normotensive | 8(10) | 8(20) | 0 | F=42.5 | 0.001 (HS) |
| Hypertensive | 54(66) | 13(39) | 41(62) | ||
| Hypotensive | 20(24) | 20(41) | 0 | ||
| BMI (Kg/m2) | |||||
| Healthy (18 – 25) | 8(10) | 7(17) | 1(20) | U=4.9 | 0.03 (HS) |
| Obese (>25) | 74(90)) | 34(42) | 40(49) | ||
| History of Pre-eclampsia | |||||
| Negative | 44(54) | 27(32) | 17(20) | U=4.6 | 0.02 (HS) |
| Positive | 38(46) | 14(37) | 24(63) | ||
| Symptoms of Pre-eclampsia | |||||
| Negative | 9(11) | 9(21) | 0 | U=10 | 0.001 (HS) |
| Positive | 73(89) | 32(40) | 41(51) | ||
| Presenting Symptoms | |||||
| Neurological | 37(51) | F=11.5 | 0.02 (S) | ||
| Oedema | 9(12) | 15(40) | 22(60) | ||
| Gastric | 18(25) | 5(57) | 4(43) | ||
| Antepartum Haemorrhage | 6(8) | 6(33) | 12(67) | ||
| Cardiac manifestations | 3(4) | 6(100) | 0 | ||
Tab. 4. Descriptive analysis of clinic-pathological features of studied groups.
| Group | Serum thrombomodulin (pg/ml) | |||
|---|---|---|---|---|
| median | range | statistics | P value | |
| All | 674 | 34 - 3414 | ||
| Pre-eclampsia | 1244 | 314- 3414 | U=0 | 0.001 (HS) |
| Control | 174 | 34 - 276 | ||
Tab. 5. Comparative analysis for serum thrombomodulin protein level between pre-eclampsia patients and healthy control group Mann-Whitney test.
| Group | Serum thrombomodulin (pg/ml) | |||
|---|---|---|---|---|
| median | range | statistics | P value | |
| Pre-eclampsia | ||||
| Mild | 674 | 314 – 1154 | U=0 | 0.0001 (HS) |
| Severe | 2294 | 1334 - 3414 | ||
| History of CS | ||||
| Negative | 1484 | 522 - 3374 | ||
| Positive | - | - | - | - |
| Number of CS | ||||
| One CS | 2054 | 522 – 3014 | F=0.02 | 0.98 (NS) |
| Two CS | 914 | 634 – 3374 | ||
| Three CS | 1514 | 874 - 2774 | ||
| Symptoms | ||||
| Negative | 614 | 314- 1154 | U=0 | 0.001 (HS) |
| Positive | 2054 | 355 - 3414 | ||
| Presenting Symptoms | ||||
| Neurological | 2074 | 434 – 3414 | F=2.9 | 0.027 (S) |
| Lower limb oedema | 914 | 374 – 3374 | ||
| Gastric manifestations | 2154 | 522 – 2774 | ||
| Anti-partum Haemorrhage | 699 | 355 – 815 | ||
| Cardiac manifestations | 2574 | 2294 - 2754 | ||
| Hypertension | ||||
| Negative | 664 | 314 – 934 | U=193 | 0.001 (HS) |
| Positive | 2154 | 374 - 3414 | ||
| Blood pressure status | ||||
| Normotensive | 554 | 314 – 874 | F=29.9 | 0.001ǂ (HS) |
| Hypertensive | 2194 | 374 – 3414 | ||
| Hypotensive | 704 | 522 - 954 | ||
| Body mass index (Kg/m2) | ||||
| Healthy (18 – 25) | 784 | 434 – 2334 | U=182 | 0.07 (NS) |
| Obese (>25) | 1754 | 314 - 3414 | ||
| History of Pre-eclampsia | ||||
| Negative | 824 | 314 – 3414 | U=570 | 0.01 (HS) |
| Positive | 2174 | 434 - 3254 | ||
Tab. 6. Comparative analysis for serum thrombomodulin protein level between mild and severe pre-eclampsia patients group Mann-Whitney test.
| Groups | Cut-off | AUC | Asymptotic 95% Confidence Interval Lower bound – Upper bound |
Biomarker sensitivity (%) |
Biomarker specificity (%) |
|---|---|---|---|---|---|
| Pre-eclampsia /Control | 904 | 0.855 | 0.76 – 0.95 | 72% | 80% |
| Mild pre-eclampsia/Severe | 1054 | 0.97 | 0.94 – 1.00 | 70% | 95% |
Tab. 7. Prognostic potential of serum thrombomodulin protein level in discrimination between mild and severe cases of pre-eclampsia; ROC curve analysis test.
Receiving operator curve illustrating the prognostic value of serum thrombomodulin in discriminating pre-eclampsia from healthy control group (Fig. 1.) and mild from severe pre-eclampsia (Fig. 2.).
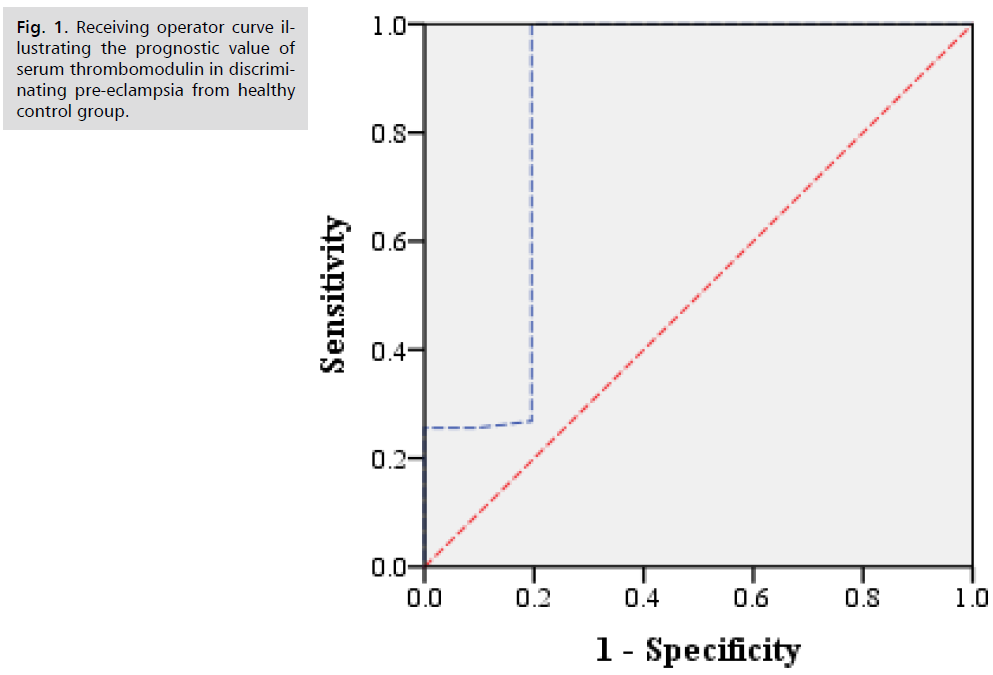
Fig 1. Receiving operator curve illustrating the prognostic value of serum thrombomodulin in discriminating pre-eclampsia from healthy control group.
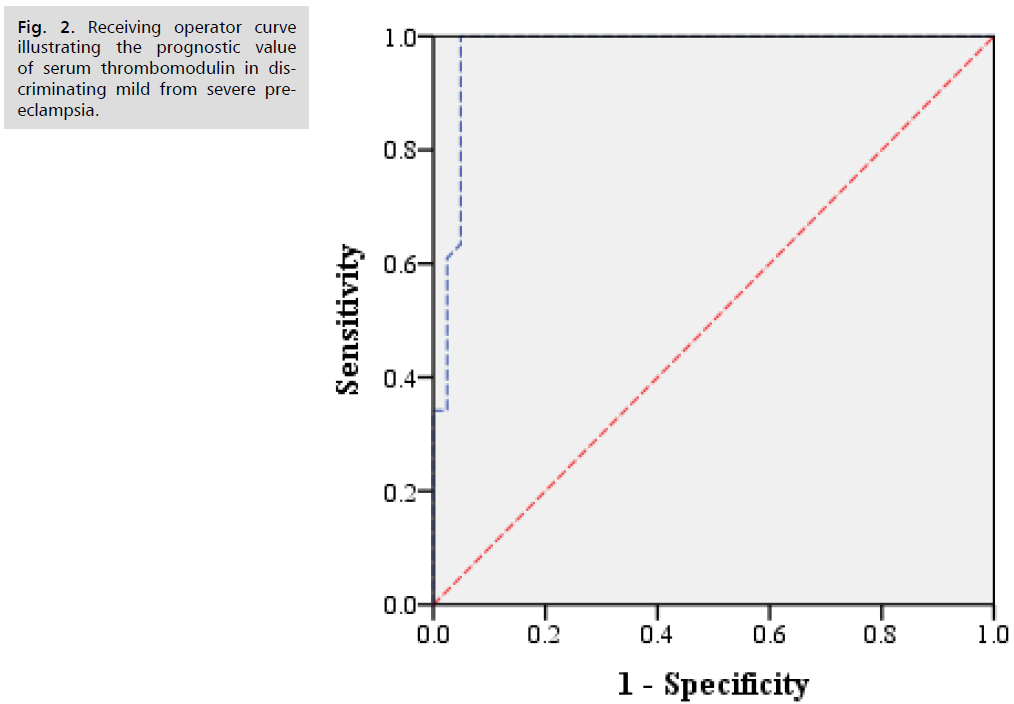
Fig 2. Receiving operator curve illustrating the prognostic value of serum thrombomodulin in discriminating mild from severe pre-eclampsia.
Preeclampsia is a multi-systemic disorder of unknown cause. Endothelial cell damage has recently been suggested to underlie the pathologic change in preeclampsia pregnancy. Thrombomodulin an endothelial cell surface glycoprotein act as a co-factor for thrombin catalysed activation of protein C. activated protein C inhibits coagulation by inactivation the coagulation factor Va and VIIIa [1].
In this study, blood samples were collected before any medical intervenion and centrifuged for 20 min at 2,000 rpm. The resulting plasma samples were stored at -70°C until assayed. Plasma TM was assayed by a one-step sandwich enzyme immunoassay using monoclonal antibodies to human TM using kits.
Regarding clinic-pathological features of pre-eclampsia patients and healthy control groups, our study found that there was high significant difference (p ≤ 0.01) between hypertensive and normal patients regarding (hypertension, obesity and history of PET).
Our study found that there was high significant difference (p ≤ 0.01) between pre-eclampsia patients and healthy control group regarding serum thrombomodulin protein level and serum thrombomodulin protein increases significantly with mild and severe preeclampsia and HELLP syndrome and considered a good marker for evaluation of hypertensive patients with pregnancy.
Herbst et al., [11] study assessed the relationships between thrombomodulin and the occurrance and persistence of preeclampsia agreed with our results and found that thrombomodulin level was higher in PET at initial evaluation (60.8 vs 41.5), delivery (64.3 vs 38.0), 24 hrPP (50.6 vs 27.5) and discharge (45.0 vs 26.9), p<0.02 each. Thrombomodulin at 24 hrPP and at discharge was higher in PET vs. non-pPET. However this study antagonized our results and regression analysis revealed thrombomodulin was not altered by maternal BMI.
Wiwanitkit, [9] study evaluated the correlation between thrombomodulin and severe preeclampsia was in line with our results and found that the overall average thrombomodulin level for the patients and controls was 66.7 + 11.9 ng/mL and 45.7 + 7.3 ng/mL, respectively. The average thrombomodulin level in the patients was significantly higher than in the controls (P <.05).
Alpoim et al., [12] study partially agreed with our study and found that TM plasma concentrations were higher in early sPE women [4782 (209–20565) pg/ml] than in late sPE women [1908 (209–6249) pg/ml] (P=0.05) and disagreed with us as there was no difference was found in TM levels when early sPE and late sPE women were compared with normotensive pregnant women [2103 (299–14063) pg/ml].
Ramireza et al., [13] study which studied the increased tissue factor and thrombomodulin expression and histopathological changes in placentas of pregnancies with preeclampsia agreed with us and found that with respect to the normalized expression of thrombomodulin, for the controls, the thrombomodulin expression level was 0.00029-fold the expression of GAPDH; in contrast, for the cases, the thrombomodulin expression level was 59.57-fold higher than the expression of GAPDH. These results demonstrate that the expression of thrombomodulin is 205413-fold greater in cases than in controls. This difference was statistically significant (p < 0.0001).
Hsu et al. [7] study evaluated the elevated circulating thrombomodulin in severe preeclampsia partially agreed with us and found that the women with severe preeclampsia but not those with mild preeclampsia, had significantly higher serum thrombomodulin levels than those in the matched control group.
Minakami et al. [14] study studied the increased levels of plasma thrombomodulin in preeclampsia agreed with us and found that plasma thrombomodulin levels were significantly higher in the women with preeclampsia (3.0 ± 1.0 ng/ml; mean ± SD) as compared with all other groups studied, i.e., normal pregnant women (2.0 ± 0.3 ng/ml), normal women in the follicular phase (1.8 ± 0.2 ng/ml) or in the luteal phase (1.8 ± 0.2 ng/ml), or in normal men (1.9 ± 0.4 ng/ml).
Bontis et al. [15] study proved that maternal plasma level of thrombomodulin is increased in mild preeclampsia agreed with us and found that plasma thrombomodulin levels were significantly higher (P < 0.001) in pregnant women with preeclampsia than in the normotensive pregnant women and the non-pregnant women.
Based on the results obtained by this study, serum thrombomodulin protein level is considered a good marker for evaluation of hypertensive patients with pregnancy and can be a suitable tool for prediction of preeclampsia.
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.