Research - (2023) Volume 0, Issue 0
Received: 21-Apr-2023, Manuscript No. gpmp-23-121970; Editor assigned: 23-Apr-2023, Pre QC No. P-121970; Reviewed: 04-May-2023, QC No. Q-121970; Revised: 19-May-2023, Manuscript No. R-121970; Published: 01-Jun-2023
Background: Preeclampsia (PE) is considered one of the leading causes of maternal and fetal morbidities. In the Egyptian population, studies were made for discovering biomarkers to help in the diagnosis of PE and in its prediction in order to avoid PE consequences through proper antenatal care. MiRNAs showed through the last decade their involvement in the pathogenesis of PE.
Objective: To investigate the levels of expression of miRNA 210 and 152 in patients with PE compared to healthy pregnant females in the third trimester.
Subjects and methods: This study was a case control study carried out in the Department of Prenatal Diagnosis and Fetal Medicine, National Research Centre, and Ain Shams University Maternity Hospital Causality Unit from January 2017 until January 2019, where the levels of expression of miRNA 210 and 152 were investigated in patients with PE. There were 20 cases, divided into 5 cases of mild PE and 15 cases of severe PE, and 10 controls of healthy pregnant females. After the miRNA extraction from the serum of the candidates, a real-time quantitative reverse-chain polymerase chain reaction has been used to estimate the Ct values of both groups.
Results: Our results showed upregulation of miRNA210 with 2.6 and 3.4 fold changes in mild and severe PE cases, respectively, with the ROC curve showing an accuracy of 80.95%, sensitivity of 82.61%, specificity of 78.95%, PTV of 82.61, AUC of 82.27, and p-value of 0.001. For miRNA152, our results showed its downregulation with a 0.1–0.4 fold change in mild and severe PE cases, respectively, with the ROC curve showing an accuracy of 67.0%, sensitivity of 73.91%, specificity of 57.89%, PPV of 68, AUC of 67, and p-value of 0.1.
Conclusion: These results show that miRNA210 is a powerful diagnostic biomarker in PE through its upregulation, and its expression is related to the severity of the disease, so it can be used as a potential predictor for PE at an earlier gestational age.
Preeclampsia; microRNAs
Pre-Eclampsia (PE) is one of the most important causes of maternal and fetal morbidity and mortality worldwide. The disease is almost exclusive to humans, and termination of the pregnancy remains the only effective treatment [1].
PE is diagnosed clinically after 20 weeks of gestation with the new onset of hypertension and proteinuria. In countries with limited access to medical care, it is estimated that PE is responsible annually for more than 60,000 deaths worldwide [2].
In 2013, the ACOG modified the diagnosis of PE so as to cover a wider range of previously missed cases by not putting proteinuria as an essential element in the diagnosis of PE in cases of the presence of signs of any end organ damage [3].
PE is a serious pregnancy-specific disease that causes maternal and fetal complications that might lead to serious morbidities and mortalities such as DIC, HELLP syndrome, hypovolemic shock, and eclampsia [4].
Regarding fetal complications, Luo and his colleagues found that the pathology causing PE will affect the blood flow reaching the placenta, thus causing fetal growth restriction, prematurity, and even cerebral palsy, as PE is associated with perinatal stroke in neonates [5].
Micro-RNAs are a class of ~22-nucleotide-long non-protein-coding RNAs that are able to regulate gene expression by binding to the 3' Untranslated Region (UTR) of target gene messenger RNA (mRNA), resulting in translational repression and/or mRNA degradation [6].
Recently, it was generally considered that ~1/3 of all human genes may be regulated by microRNAs by playing a key role in cellular activities, including cell proliferation, apoptosis, and immune responses [7].
The importance of aberrant microRNA (miR) expression in PE was first reported in 2007 [8].
Two miRNAs (miR-152 and miR-210) showed significant elevation in pre-eclamptic sera starting with the second trimester [9].
MicroRNA-210 (miR-210) is considered one of the most prevalent PE-associated miRNAs, according to [10].
Despite the improvements in the prediction of the disease, there is no treatment that reverses this pathology once it has begun; the only effective treatment for PE is termination of pregnancy regardless of the gestational age of the pregnancy, which stresses the need for discovering novel biomarkers for PE to be used in its diagnosis and to be implemented in its prediction in the first trimester [11].
This study investigated the levels of expression of miRNA 210 and 152 in patients with PE compared to healthy pregnant females in the third trimester.
We performed a case control study after the approval of both ethical committees (Ain Shams University registration number MD339/2016 and National Research Center registration number 16458) carried out in the Department of Prenatal Diagnosis and Fetal Medicine, National Research Centre, and Ain Shams University Maternity Hospital Causality Unit from January 2017 until January 2019. The study included two groups of 30 women, with matched criteria according to inclusion and exclusion criteria.
Inclusion criteria were gestational age > 34 weeks calculated from the onset of a reliable and sure LMP. If not available, a late first trimester ultrasound was used for estimation of the gestational age, and the date of last delivery was one year apart (non-pregnant group).
Exclusion criteria were medical disorders associated with pregnancy, including chronic hypertension, diabetes mellitus, and multiple fetal pregnancies.
The first group was 20 cases of PE, which were grouped into 5/20 (25% of cases) with mild PE and 15/20 (75% of cases) with severe PE. The cases of PE were selected according to the American College of Obstetrics and Gynecology guideline for blood pressure above 140/90 mm/hg on two different occasions (6 hours apart) under complete physical or mental rest, or blood pressure above 160/110 mm/hg in a single reading. Proteinuria above 300mg in 24-hour urine collection or urine dipsticks plus (+) If adverse complications of PE (signs of end organ damage) appear, proteinuria is not needed for the diagnosis of PE [12].
The control group of our study was 10 pregnant females with healthy pregnancies without any of the criteria of PE or a history of hypertensive disorders during pregnancy. All pregnant females received full counseling before and after each step of the procedures, including a complete history taking, including personal history, present history, and detailed obstetric history of complications in the past or current pregnancy, the last menstrual period for a proper assessment of gestational age, a reliable first trimester ultrasound, a detailed family history and pedigree analysis, a general examination and assessment of weight, height, and body mass index, and urine dipsticks for proteinuria.
Third trimester ultrasound (above 34 weeks)
All subjects were examined by 2D ultrasound for a detailed anomaly scan, assessment of fetal growth for detection of (FGR) and (SGA), and assessment of the liquor for detection of oligohydramnios amniotic fluid index (A.F.I<5).
Laboratory evaluation included liver function tests (AST, ALT), kidney function tests (serum creatinine), coagulation profiles (PT, PTT, and INR), and a complete blood picture.
Detection of maternal serum microRNA (210 and 152)
A total of 5 mL of blood samples were withdrawn from all pregnant females in both groups of the study in the third trimester (above 34 weeks of gestation). In which miRNA extraction was done by the miRNeasy isolation kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and reverse transcripted into complementary DNA (c DNA) using the TaqMan MicroRNA kit containing microRNA-specific stem-loop RT primers and the TaqMan MicroRNA Reveres transcription kit (Applied Biosystems, USA), quantification was recorded as the PCR-CT.
Statistical analysis
The data was analyzed using Microsoft Excel 2010 and the statistical package for social science (SPSS version 24.0) for Windows (SPSS IBM, Chicago, IL). Continuous normally distributed variables were represented as mean ± SD with a 95% confidence interval, while nonnormal variables were summarized as median with a 25 and 75 percentile, and using the frequencies and percentages for categorical variables, a p value < 0.05 was considered statistically significant. To compare the means of normally distributed variables between groups, the Student’s t test was performed, and the Mann-Whitney test was used for non-normal variables. To compare the median of non-normally distributed variables between groups, the χ2 test or Fisher’s exact test was used to determine the distribution of categorical variables between groups.
The diagnostic performance of hsa-miRNA152 and hsa-miRNA210 was assessed by receiver operating characteristic (ROC) curves. The area under the ROC (AUROC) was used as an index to compare the accuracy of tests. The cut-off for the diagnosis of the group in the study was taken from the point of maximum combined sensitivity and specificity. The sensitivity and specificity of relevant cut-offs were also displayed. Spearman's rank correlation coefficient (r) was used to show the correlation between different parameters in this study. Effect modifications were evaluated by stratification, and statistical interaction was assessed by including the main effect variables and their product terms in the logistic regression model.
Family history and past history were significant with p-values of 0.01 and 0.04, respectively, as shown in Tab. 1. All control pregnant females had blood samples taken for laboratory investigations, as mentioned above, and albumin was checked. All laboratory results were of normal value and negative for albumin. All subjects had a detailed anomaly scan at the time of sample withdrawal, with no anomalies present, as shown in Tab. 2. Parity (PG) was significant with a p-value of 0.01 between mild and severe PE cases, as shown in Tab. 3. The 1st P value is comparing between control and mild P.E., while the 2nd is comparing between control and severe P.E., and the 3rd is comparing mild P.E. and severe P.E. P. value bearing (#) initial is significantly different comparing between control and mild P.E.
| Control n=10 |
Case (PE) n=20 |
p. value | |||
|---|---|---|---|---|---|
| Demographic | Age | (24-30) 27.8 ± 3.1 | (16-46) 31.2 ± 7.8 | 0.5 | |
| Family History (P.E.) | N | 10(100%) | 17(85%) | 0.01* | |
| P | 0(0%) | 3(15%) | |||
| Past History | N | 10(100%) | 18(90.0%) | 0.04* | |
| P | 0(0%) | 2(10.0%) | |||
| Obstetric | Parity | PG | 2(20%) | 6(30%) | 0.03* |
| Other | 8(80%) | 14(70%) | |||
| Gest.age/day | 264.9 ± 7.4 | 254.4 ± 13.5 | 0.1 | ||
| Clinical examination | B.M.I | 30.5 ± 3.3 | 32.0 ± 6.0 | 0.8 | |
| Blood Pressure(mm/hg) | 107/72 ± 12.3/8.2 | 160.7/103.3 ± 13.8/9.5 | 0.01* | ||
| U/S | A.F.I | 9.9 ± 2.1 | 8.7 ± 2.3 | 0.3 | |
Tab. 1. Demographic and clinical characteristics of control and cases.
| Control n=10 |
Case (PE) n=20 |
||
|---|---|---|---|
| Mode of delivery | C/S | 3(30%) | 17(85%) |
| SVD | 7(70%) | 3(15%) | |
| Neonatal gender | F | 6(60%) | 9(45%) |
| M | 4(40%) | 1(55%) | |
| Outcome(Kg) | 2.8 ± 0.2 | 2.3 ± 0.9 | |
| APGAR/score | 7.5 ± 1.0 | 7.1 ± 1.4 | |
| F=Female; M=Male | |||
Tab. 2. Outcome of control and cases.
| Mild P.E n=5 |
Severe P.E n=15 |
p. value | |||
|---|---|---|---|---|---|
| Demographic | Age | 36.2 ± 3.7 | 29.8 ± 1.7 | 0.1 | |
| Family History (P.E.) | N | 4 (80.0%) | 13 (86.7%) | 0.6 | |
| P | 1 (20.0%) | 2 (13.3%) | |||
| Obstetric | Parity | PG | 0 (0.0%) | 6 (40.0%) | 0.001** |
| Other | 5 (100.0%) | 9 (60.0%) | |||
| Gest.age/day | 264.0 ± 8.0 | 252.0 ± 3.0 | 0.2 | ||
| Clinical examination | B.M.I | 32.6 ± 2.9 | 31.9 ± 1.4 | 0.8 | |
| Blood Pressure(mm/hg) | 149.0/98.0 ± 4.0/2.0 | 164.0/105.0 ± 3.0/2.4 | 0.01* | ||
| Lab. Investigations | Albumin(+) | 1.0 ± 0.01 | 1.7 ± 0.2 | 0.002** | |
| HGBg/dl | 11.8 ± 0.4 | 11.3 ± 0.3 | 0.3 | ||
| Platelets | 252.8 ± 10.6 | 186.0 ± 21.4 | 0.01* | ||
| AST | 24.4 ± 2.9 | 29.2 ± 4.3 | 0.3 | ||
| ALT | 17.4 ± 2.0 | 23.6 ± 4.4 | 0.2 | ||
| S. Creat. | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.7 | ||
| PT | 12.3 ± 1.1 | 11.9 ± 0.8 | 0.5 | ||
| PTT | 28.9 ± 3.2 | 32.0 ± 3.2 | 0.1 | ||
| INR | 1.0 ± 0.1 | 1.0 ± 0.01 | 0.2 | ||
| U/S | A.F.I | 9.0 ± 1.6 | 8.6 ± 0.5 | 0.8 | |
| Mode of delivery | C/S | 4 (80.0%) | 13 (86.7%) | 0.6 | |
| SVD | 1 (20.0%) | 2 (13.3%) | |||
| Neonatal gender | F | 3 (60.0%) | 6 (40.0%) | 0.5 | |
| M | 2 (40.0%) | 9 (60.0%) | |||
| Outcome(Kg) | 2.5 ± 0.4 | 2.2 ± 0.2 | 0.4 | ||
| APGAR/score | 7.2 ± 0.5 | 7.1 ± 0.34 | 0.8` | ||
Tab. 3. Demographic and clinical characteristics of PE cases.
Value bearing (†) initial is significantly different comparing between control and severe P.E.
1 initial p value <0.01 is significant, and 2 initial sp values <0.001 are highly significant, as shown in Tab. 4. and Fig. 1.
| Biomarkers | Pregnant Control N=10 |
P.E Patient n=20 |
p. value | |
|---|---|---|---|---|
| Mild P.E n=5 |
Severe P.E n=15 |
|||
| miRNA152 | 30.5 ± 2.8 | 37.2 ± 3.4 | 34.1 ± 2.7 | 0.01# 0.001†† 0.9 |
| miRNA210 | 33.8 ± 1.7 | 35.0 ± 2.7 | 34.9 ± 3.7 | 0.4 0.3 0.1 |
Tab.4. Biomarkers results in the studied groups.
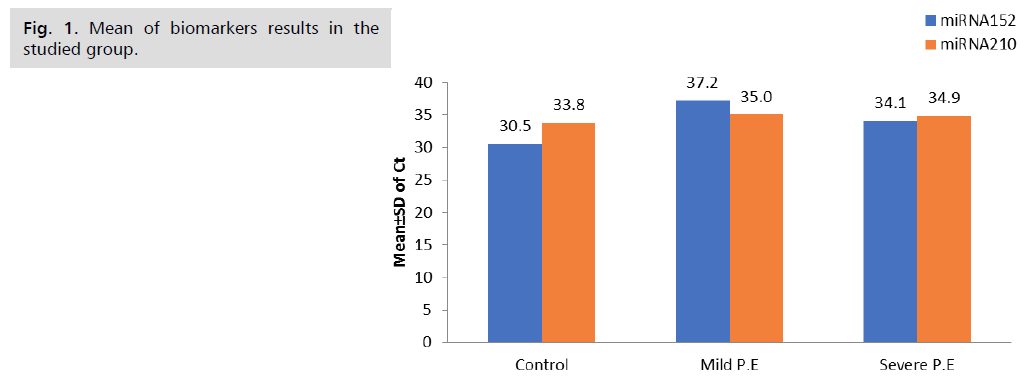
Fig 1. Mean of biomarkers results in the studied group.
The fold change results depend on the fold change. Fold-Change (2^(- Delta Delta Ct)) is the normalized gene expression (2^(- Delta Ct)) in the test sample divided by the normalized gene expression (2^(- Delta Ct)) in the control sample. (Fold-change values less than one indicate a negative or down-regulation, as shown in Tab. 5. and Fig. 2.) miRNA 210 shows a significant p-value of 0.001, while miRNA 152 shows a non-significant p-value of 0.1, as shown in Tab. 6. and Fig. 3. miRNA 210 shows a significant p-value of 0.001, while miRNA 152 shows a non-significant p-value of 0.5, as shown in Tab. 7.
| Biomarkers | Pregnant Control N=10 |
P.E Patient n=20 |
|||
|---|---|---|---|---|---|
| Mild P.E n=5 |
Severe P.E n=15 |
||||
| Fold-change | Type of Regulation | Fold-change | Type of Regulation | ||
| miRNA152 | 1 | 0.1 | Down | 0.4 | Down |
| miRNA210 | 1 | 2.6 | Up | 3.4 | Up |
Tab. 5. Biomarkers fold-change in the studied groups.
| Markers | Cutt-off | Sensitivity | Specificity | PPV | Accuracy | AUC | 95% C.I | p-value |
|---|---|---|---|---|---|---|---|---|
| miRNA152 | ≥ 33.7 | 73.91 | 57.89 | 68.0 | 67.0 | 60.0 | 4.0-75.0 | 0.1 |
| miRNA210 | ≥ 32.98 | 82.61 | 78.95 | 82.61 | 80.95 | 82.27 | 63.40-91.89 | 0.001** |
Tab. 6. Diagnostic performances of biomarkers to discriminate patients than control cases.
| Biomarker | OR | 95% C.I | P-value |
|---|---|---|---|
| miRNA152 | 1.09 | 0.84 – 1.43 | 0.5 |
| miRNA210 | 1.64 | 1.206 –2.24 | 0.001** |
Tab. 7. Univariate analysis showing the predictive power of different biomarkers for patient diagnosis.
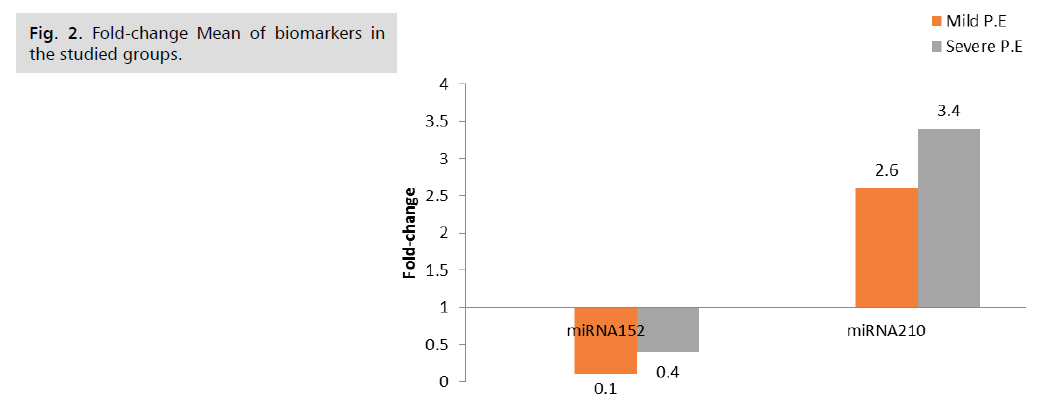
Fig 2. Fold-change Mean of biomarkers in the studied groups.
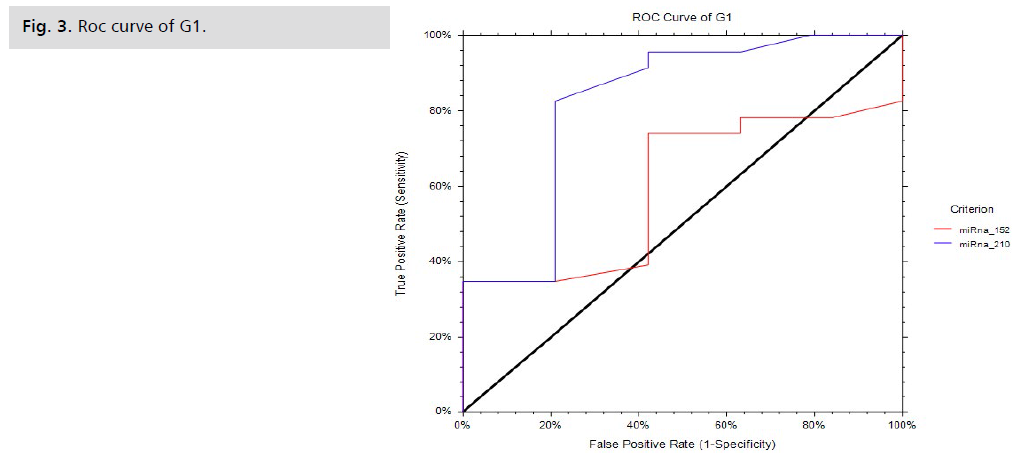
Fig 3. Roc curve of G1.
The demographic data of the pre-eclamptic cases in comparison to the control group shows the appearance of PE in the extremes of age [(16–46) years], which is coincident with Duckitt, who showed that the extremes of age were found to be a risk factor for the development of PE, which was expressed by a J-shaped curve for the relationship between the maternal age and the incidence of PE, with a slightly increased pattern among young pregnant with an increase in severity of PE and a markedly increased incidence among women of more than 35 years [13]. Family history was found to be a major risk factor for PE, with a significant p-value of 0.01. This is a co-incident with Heather, in which he showed that maternal family history was 1.49 times the risk compared to patients without a maternal history of PE, and the history of any female relative to the patient increased the risk by 150%. Past history showed significance in the reoccurrence of PE with a significant p-value of 0.04, which is in coherence with Heather and his colleagues, where previous PE increased the recurrence by 25.2 times [14].
Regarding parity, the PE group had 6 cases (30% of total cases) with a p-value of 0.03, which is consistent with Essam and his colleagues’ belief that parity is considered one of the risk factors for PE, where it has been shown that PE is more common and aggressive in the primi gravidae compared to the multigravidae [15].
Regarding the BMI in our study, there is no statistical significance between the case and control groups with a p-value of 0.8. The demographic data of the pre-eclamptic cases show no Primi Gravidae (PG) cases were included in mild PE, while it formed 6/15 cases (40%) with severe PE with a significant p-value of 0.001, which is coincident with the study done by Shikanova, as they found that PE in PG is more of a severe type with an adverse outcome and complications such as renal failure, intracranial hemorrhage, and IUFD [16].
Platelet count and level were significant by being higher in mild PE with a mean of 252.8±10.6, 186.0±21.4 in severe PE with a p-value of 0.01, which is in agreement with the study done by Zhang and his colleague, who stated that the changes in parameters of the platelets can identify PE and atypical PE, which is associated with the absence of high blood pressure or proteinuria and indicating HELLP syndrome [17].
Liver enzymes (AST and ALT) showed no significant difference between mild and severe PE with p-values of 0.3 and 0.2, respectively; liver enzymes increased in cases of complicated severe PE, such as HELLP, where its incidence was 10–20% [18].
In a case-control study done by Yi and his colleagues on 15 mild cases with PE and 15 severe cases with PE he found that miRNA 210 was evaluated for both groups in comparison to the healthy pregnant control group. The results correlated with our study, where miRNA 210 was upregulated with a fold change of 3.27 in mild PE and 9.49 fold change in severe PE with a p-value less than 0.001 [19].
Having similar results compared to our study, Gan, et al. showed that miRNA 210 had a diagnostic significance in PE with an AUC of 0.75 and a p-value of 0.1 [20].
As for miRNA 152 in our study, it has an accuracy of 67.0%, a sensitivity of 73.91%, a specificity of 57.89%, a PPV of 68, an AUC of 67, and a p-value of 0.1, making it a non-significant biochemical marker in the diagnosis of PE.
In our study, miRNA 210 was found to be expressed more in severe PE than mild PE according to the univariate analysis, which shows the significance of miRNA 210 for the prognosis of PE with an OR of 1.64, a 95% confidence interval of 1.206-2.24, and a p-value of 0.001. Thus, the greater the severity of PE and complications, the greater the expression of miRNA 210, as it has a direct correlation with hypoxia. MiRNA 152 in our study had an OR of 95%C.I. =0.84-1.43 and a p-value of 0.5, thus making it non-significant in the prognosis of PE.
Fasanaro, et al. observed in their study the same findings: miRNA 210 was upregulated more than 35 fold compared to the normoxic control healthy pregnant group at 48 hours of delivery, and the levels of miRNA 210 were maintained in the blood of patients at such high levels for 72 hours even after termination of pregnancy [21].
Our study showed that miRNA210 is a significant biomarker for cases of PE in the Egyptian population, being upregulated with an accuracy of 80.95%, sensitivity of 82.61%, specificity of 78.95%, PBV of 82.61, AUC of 82.27, and p-value of 0.001, being more expressed in complicated cases of PE.
The authors would like to thank Ain Shams University.
No funding sources.
None declared.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.