Research - (2022) Volume 0, Issue 0
Efficacy and safety of different doses of vaginal misoprostol prior to intra uterine contraceptive device insertion: A randomized three arms double-blind clinical trial
Mohamed H. Salama1, Shaimaa G. Helmy1, Mohamed E. Elhodiby2, Mohammed A. El-Kadi1 and Maii Nawara1*Received: 23-Aug-2022, Manuscript No. gpmp-22-75755; Editor assigned: 24-Aug-2022, Pre QC No. P-75755; Reviewed: 07-Sep-2022, QC No. Q-75755; Revised: 15-Sep-2022, Manuscript No. R-75755; Published: 29-Sep-2022
Abstract
Objective: To evaluate the efficacy and safety of different doses of vaginal misoprostol prior to intrauterine contraceptive device (IUD) insertion among women who had delivered by elective cesarean section (CS: no history of vaginal delivery).
Study Design: A randomized, double-blind, placebo-controlled trial.
Setting: Conducted in the Family Planning Clinic of Ain Shams University Maternity Hospital, Egypt, for a 3-month period.
Methods: Women (n=180) who wished insertion of copper IUD after elective CS were equally divided into three groups: Group 1 received 200 mcg misoprostol, Group 2 received 100 mcg misoprostol, and Group 3 received placebo. Misoprostol was administered vaginally, 3 hours before the IUD insertion. The primary outcome was pain scores (a 10 cm visual analogue scale (VAS)) within 5 minutes of IUD insertion. The secondary outcome was a 10-grade provider ease of insertion score.
Results: Group 1, compared with Group 2 and 3, showed significantly lower VAS score, VAS was significantly lower in Group 1 (1.7 ± 0.8) than Groups 2 and 3 (3.6 ± 0.9 and 3.7 ± 0.9, respectively), with no statistical significant difference between Groups 2 and 3. Mean ± SE (95% CI) for differences between Groups 1&3, Groups 2&3, and Groups 1&2 was - 2.0 ± 0.2 (-2.3–-1.7), 0.0 ± 0.2 (-0.4–0.3), and -2.0 ± 0.2 (-2.3–-1.6), respectively. Conclusion: 200 mcg vaginal misoprostol, compared with 100 mcg, offered better efficacy with no significant increase in the adverse effects.
Keywords
Misoprostol; IUD; Contraception
Introduction
Intrauterine contraceptive device (IUD) is the most widely used reversible form of contraception worldwide [1]. Anticipated insertion pain and healthcare providers’ concerns about difficult insertion are the major limitations against IUD use. Effective methods to ease IUD insertion and overcome obstacles hindering IUD use are important [2]. When compared to women who delivered vaginally, nulliparous women and those who delivered by cesarean section only suffer more from failure of insertion due to a narrower cervical os, yet, the failure rate is still low [3,4].
Misoprostol is a synthetic prostaglandin E1 analogue. It has uterotonic and cervical ripening effects. This leads to using misoprostol in numerous gynecologic procedures [5]. Previous reports in the literature about using misoprostol before IUD insertion are contradictory. Six trials compared between 400 μg misoprostol and placebo in nulliparous women. Five of them indicated that misoprostol did not help with pain [6]. Moreover, nine RCTs examined the effect of misoprostol on provider ease of insertion, and seven found no significant differences between study groups [7]. Others found an easier insertion after its use but no difference in pain [8-10]. As there is still no consensus in the literature regarding the administration of misoprostol before IUD placement, the present study aims at evaluating the efficacy and safety of different doses of misoprostol before IUD insertion among women who delivered only by elective cesarean section.
Methods
Study Design: This is a randomized, single center, placebo-controlled, three-arm double-blinded clinical trial. The study participants were equally randomized to the three intervention groups.
Study Setting: The current study was conducted in the Family Planning Clinic of Ain Shams University Maternity Hospital, Egypt. The Medical Ethical Review Board of Ain Shams University approved the study.
Participants: All women who came to the Family Planning Clinic seeking an IUD insertion during the study period were clinically evaluated and invited to participate in the study if they did not have any contraindications for IUD insertion in line with World Health Organization Medical Eligibility Criteria (WHO-MEC) [11]. We included non-pregnant women, aged 18–45 years, who have delivered only by elective cesarean section, and did not receive any analgesics in the 24 hours before the IUD insertion. Women with abnormal uterine bleeding, any known uterine abnormalities, spasmodic dysmenorrhea, chronic pelvic pain, or history of cervical surgery were excluded. Additionally, we excluded women who were allergic to misoprostol and NSAIDs or refused to participate in the study. Before participating in the study, all eligible women signed written informed consent after explaining the nature of the study to them.
Patient and Public Statement: The eligible patients were invited to participate in the study through consenting to undergo the study intervention, after full explanation of the study objectives and procedure.
Interventions: We allocated the participants into one of the 3 groups (60 patients each) according to a computer-generated random sequence. Group 1 received 1 tablet (200 mcg) of misoprostol and 1 placebo tablet; Group 2, received 1 tablet (100 mcg) of misoprostol and 1 placebo tablet; Group 3, received 2 placebo tablets. Misoprostol was obtained from Sigma Company (Monufia, Egypt).
Three hours before IUD insertion, the tablets were inserted, through digital vaginal examination, by the investigator into the posterior vaginal fornix while the woman was lying in the lithotomy position. This pre-procedure interval was suggested by some previously conducted studies [12]. Ketoprofen, 100 mg suppository was inserted rectally to every participant 3 hours before IUD insertion. The IUD was placed according to the recommendations of the manufacturer. Each woman received a copper T380A-IUD (Pregna®, DKT, Egypt). Insertion was performed within 1-2 days after cessation of menses.
Outcomes: The primary outcome measured was the pain scores in the three groups. The secondary outcomes included a 10-grade provider ease of insertion score, percentage of successful insertions, the women's level of satisfaction at the end of insertion, need for additional analgesia after the insertion, the side effects of the drug, and complications from the insertion.
Pain severity was measured using a 10 cm visual analogue scale (VAS), where 0 means no pain and 10 means worst imaginable pain [13]. The VAS score was assessed within 5 minutes of insertion. Although this might result in lower pain scores, due to the relief experienced by the patient as the IUD is finally inserted, we thought that it would not have been very ethical or practical to ask the participants about it at the actual time of the IUD insertion.
Complications from IUD insertion such as uterine bleeding, perforation, cervical trauma, and failure of insertion were recorded. The duration was recorded starting from putting the loaded applicator at the external os till withdrawal of the applicator after IUD insertion. After insertion, the ease of IUD insertion was reported by the physician using the ease of insertion score (ES), which was calculated at a graduated VAS-like scale from 0 to 10, where zero means very easy insertion and ten means terribly difficult insertion.
Level of satisfaction with IUD insertion was expressed by the women through choosing a grade at a 10 cm VAS-like scale, where 0 means absolute non-satisfaction and 10 means maximum satisfaction. Finally, all women were asked if they need further analgesia after 15 min of the procedure. Side effects of the medications encountered by the participants were also reported.
Randomization, allocation, and blinding: Randomization of the study participants was carried out using a computer-generated list of random numbers that was confidentially kept with the study pharmacist. The pharmacist packed the two tablets for each participant of the 3 study groups into a sealed envelope. The randomization table indicated which study group tablets should be packaged into each of the serially numbered envelopes. Thus, the study investigator had only two tablets in each envelope, with all tablets (200, 100 mcg misoprostol, and placebo) identical in shape, size, color, and weight. This achieved both investigator and participant blinding. The investigator documented the envelope number in the data collection form, along with the other details. Only at the end of the study, the envelope allocation to the study groups was revealed for the statistician.
Sample size calculation: Sample size was calculated using PASS 11 program for sample size calculation. Assuming Mild/absent difficulty of insertion in misoprostol and placebo groups 73.3% and 45.2% respectively. This fig. 1. was derived from previous study [10]; a sample size of 60 patients in each group achieves 90% power to detect this difference with a significance level 0.05
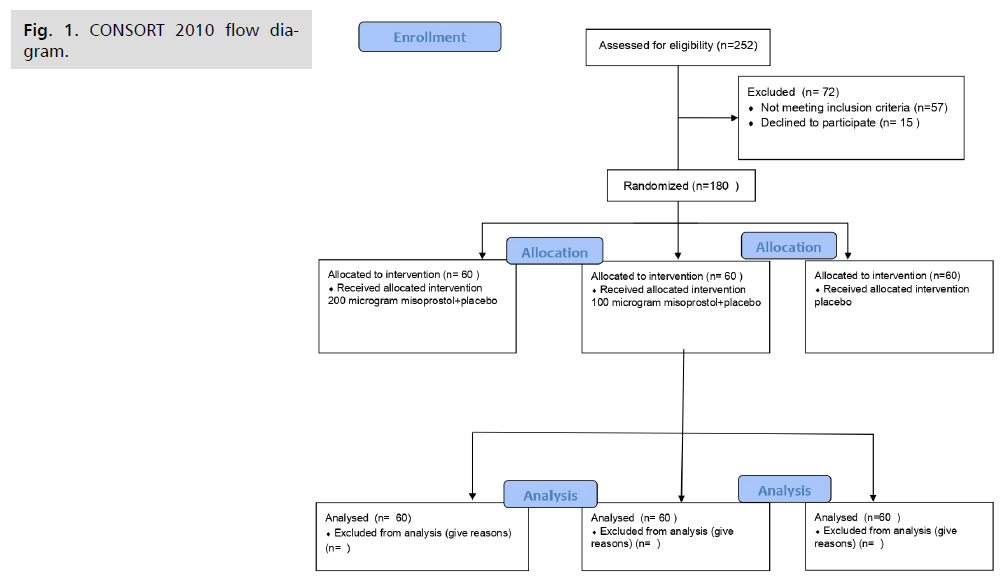
Fig 1. CONSORT 2010 flow diagram.
Statistical analysis: The collected data was coded, tabulated, and statistically analyzed using IBM SPSS statistics (Statistical Package for Social Sciences) software, version 22.0 (IBM Corp., Chicago, USA, 2013) and Microsoft Office Excel 2007. Quantitative data was described as mean ± SD (standard deviation) and was compared using ANOVA test, while its effect size was expressed as mean ± SE (standard error) and 95% confidence interval. Qualitative data was described as numbers and percentages and was compared using Chi square test. Fisher’s exact test was used for variables with small expected numbers. Post hoc Bonferroni test was used to find out sources of differences, while its effect size was expressed as relative rate and 95% confidence interval. The level of significance was taken at a significant P value < 0.050; otherwise, it is nonsignificant.
Results
We enrolled participants from March to June 2020. For details of recruitment, randomization and intervention, see CONSORT flow diagram. The three groups were similar regarding the baseline characteristics with no statistically significant difference (Tab. 1.).
| Group 1 200 (n=60) |
Group 2 100 (n=60) |
Group 3 (placebo) (n=60) |
Test | P value | ||
|---|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 31.12 ± 4.88 | 29.93 ± 4.56 | 30.41 ± 4.70 | F=0.9233 | 0.387 |
| Range | 23-44 | 22-43 | 23-45 | |||
| BMI (kg/m2) |
Mean ± SD | 26.82 ± 4.11 | 27.78 ± 4.48 | 25.92 ± 4.2 | F=2.852 | 0.06 |
| Range | 19.8-34.4 | 20.1-33.9 | 20-33.8 | |||
| Parity | Median | 1 | 1 | 2 | KW=3.884 | 0.143 |
| Range | 1-4 | 1-3 | 1-4 | |||
Tab. 1. Demographic data of the studied population.
The pain scores reported by women were significantly lower in Group 1 (1.7 ± 0.8) than Groups 2 and 3, with no statistical significant difference between Groups 2 and 3 (Tab. 2.).
| Group 1 (n=60) |
Group 2 (n=60) |
Group 3 (n=60) |
Test | P value | ||||
|---|---|---|---|---|---|---|---|---|
| VAS | Median | 1.5 | 3 | 3 | KW = 94.378 |
<0.001* | P1 | <0.001* |
| Range | 1-4 | 2-5 | 2-5 | P2 | <0.001* | |||
| P3 | 0.998 | |||||||
| ES | Median | 2 | 4 | 4 | KW = 116.35 |
<0.001* | P1 | <0.001* |
| Range | 1-4 | 2-6 | 2-6 | P2 | <0.001* | |||
| P3 | 0.121 | |||||||
Tab. 2. Visual analogue scale (VAS) and Ease score (ES) of all studied groups.
The ease of insertion scores (ES) reported by the physician after insertion were lower in Group 1 (1.6 ± 0.7) than Groups 2 and 3 (3.9 ± 1.0 and 4.3 ± 0.8, respectively), with no statistical significant difference between Groups 2 and 3 (Tab. 3.). Failure of IUD insertion was encountered in two cases in Group 1 versus three cases in Group 2 and five cases in Group 3, which was not statistically significant (Tab. 3.). Duration of insertion (seconds) was significantly lower in Group 1 (14.3 ± 1.0) than Groups 2 and 3 (15.5 ± 0.7 and 15.3 ± 1.3, respectively) (Table 3). A higher level of satisfaction from the whole procedure was reported in participants of Group 1 (mean 8.6 ± 0), compared to Groups 2 and 3 (6.0 ± 0 and 5.6 ± 1, respectively), with no statistical significant difference between Groups 2 and 3 (Tab. 3.). Regarding additional analgesia, more participants in Groups 2 and 3 needed further doses (23 (38.3%) and 22 (36.7%), respectively) compared to Group 1 (5 (8.3%)) (Tab. 3.). No side effects occurred in all groups apart from nausea that occurred in one patient (1.7%) in Group 1 and vomiting that also occurred in one patient (1.7%) in the same group. Abdominal cramps were encountered only in patients of Group 1 (11.7%). No complications related to insertion occurred in all groups, such as perforation, cervical trauma, or uterine bleeding (Tab. 3.).
| Outcomes | Group 1 (n=60) |
Group 2 (n=60) |
Group 3 (n=60) |
P value | Groups 1 Vs 3 |
Groups 2 Vs 3 |
Groups 1 Vs 2 |
|---|---|---|---|---|---|---|---|
| Mean ± SE, 95% CI | |||||||
| ES, Mean ± SD | 1.6 ± 0.7a | 3.9 ± 1.0b | 4.3 ± 0.8b | ^<0.001* | -2.6 ± 0.1 -2.9–-2.3 |
-0.3 ± 0.2 -0.7–0.0 |
-2.3 ± 0.2 -2.6–-2.0 |
| Duration of Insertion (seconds), Mean ± SD | 14.3 ± 1.0a | 15.5 ± 0.7b | 15.3 ± 1.3b | ^<0.001* | -1.0 ± 0.2 -1.4–-0.6 |
0.2 ± 0.2 -0.2–0.6 |
-1.2 ± 0.2 -1.5–-0.9 |
| Satisfaction, Mean ± SD | 8.6 ± 0.7a | 6.0 ± 0.9b | 5.6 ± 1.0b | ^<0.001* | 3.0 ± 0.2 2.6–3.3 |
0.3 ± 0.2 0.0–0.7 |
2.6 ± 0.2 2.3–2.9 |
| Relative risk (95% CI) | |||||||
| Successful IUD insertion, (n, %) | 58 (96.7%) | 57 (95%) | 55 (91.7%) | §0.610 | 1.05 (0.96–1.15) |
1.04 (0.94– 1.14) |
1.02 (0.94–1.10) |
| Need of additional analgesia, (n, %) | 5 (8.3%) a | 23 (38.3%) b | 22 (36.7%) b | #<0.001* | 0.23 (0.09–0.56) |
1.05 (0.66– 1.66) |
0.22 (0.09–0.53) |
| Nausea, (n, %) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | §0.999 | NA | NA | NA |
| Vomiting, (n, %) | 1 (1.7%) | 0 (0.0%) | 0 (0.0%) | §0.999 | NA | NA | NA |
| Abdominal pain, (n, %) | 7 (11.7%) a | 0 (0.0%) b | 0 (0.0%) b | §0.001* | NA | NA | NA |
Tab. 3. Secondary outcomes of all studied groups.
Discussion
The role of misoprostol before IUD insertion has been reported by many trials [6-10]. The results of Bakas et al. demonstrated that doses of 400 mcg and 200 mcg of vaginal misoprostol before IUD insertion had comparable results. They proposed that a dose of 200 mcg may be better to avoid unnecessary usage of a higher dose, to avoid the potential side impacts [14]. This was also proposed by Alanwar et al, who recommended the use of a lower dose of misoprostol [15]. Following on the same principle, we performed our study aiming to explore the safety and efficacy of even lower doses of misoprostol (100 mcg).
Our results indicated that 200 mcg misoprostol was associated with lower pain scores (mean 1.7 ± 0.8) versus 100 mcg misoprostol and placebo (3.6 ± 0.9 and 3.7 ± 0.9, respectively). The VAS values reported in our study appear to be lower than those reported in other studies (2.7 ± 0.6 and 4.3 ± 0.8) [8]. These lower values of the VAS score may be explained by the pre-emptive use of rectal ketoprofen (100 mg), which might have reduced the pain perception in the study subjects. Abbas et al., revealed that the use of 150 mg oral ketoprofen, before copper IUD insertion, significantly reduced the pain scores during insertion [16]. The findings of our study demonstrated that a dose of 200 mcg misoprostol was associated with better ease of insertion scores and lower need for additional analgesia. Several studies concluded that 200, 400, or 1000 µg of vaginal misoprostol preoperatively was superior to placebo [17-21]. Contrary to our results, Swenson et al., comparing the effects of self-administered misoprostol versus placebo before IUD insertion, failed to show any difference in the easiness of insertion, although neither the doctors nor the patients were blinded in that study. Moreover, self-administration of misoprostol vaginally may not be very effective. These small tablets are better administrated as deep as possible by the gynecologist [22].
Additionally, a systemic review, conducted by Lopez et al., indicated that misoprostol did not decrease the pain score at IUD insertion. However, most of the included studies in this review used misoprostol buccally or sublingually, and again; these were self-administered [6].
In our study, no significant difference among the three groups as regards successful IUD was observed. In agreement with our findings, Bahamondes et al. revealed that there were no significant differences between the misoprostol group and the control group as regards successful IUD insertion [2].
The overall failure rate in our study is 5.5% (10 cases). This figure is slightly higher than those reported in other studies (3.3%) [5]. This higher incidence may be attributable to the fact that our study included exclusively women who have never had vaginal delivery or attempted vaginal delivery.
Participant satisfaction was higher in Group 1 than Groups 2 and 3, but there was insignificant difference between Group 2 and Group 3. Similarly, Abdellah et al. found that the level of satisfaction with the entire procedure was higher in the misoprostol group [8].
The incidence of abdominal cramps was higher in women who received the 200 mcg dose, while there was insignificant difference among the three groups as regards nausea and vomiting. The greater frequency of cramps results from increased uterine contractility caused by misoprostol, which is a strong prostaglandin [23]. However, these cramps did not seem to be troublesome to the patients as they did not request further analgesia.
The strengths of our study include proper randomization and careful double blinding, as all tablets were identical. The insertion was performed by the same provider for all participants. Finally, the study was conducted on a homogenous cohort of women who have delivered only by elective cesarean section.
The limitations of the study include the use of VAS. It is to be noted that the outcome for all studies assessing pain scores is subjective and may be affected by many factors. The pre-emptive use of ketoprofen in our study could have affected the pain score associated with IUD insertion and might have decreased the cramps related to misoprostol use.
Conclusion
In conclusion, a dose of 200 mcg misoprostol inserted vaginally, along with rectal ketoprofen premedication, seemed to achieve the best balance of efficacy and safety in women who have only delivered by cesarean section. Attempt to further lower the dose to 100 mcg led to lower efficacy, with no added benefit regarding the side effect profile.
Authors Contribution
(A) Study Design · (B) Data Collection. (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- d'Arcangues C. Worldwide use of intrauterine devices for contraception. Contraception. 2007;75(6):S2-S7.
- Bahamondes MV, Espejo-Arce X, Bahamondes L. Effect of vaginal administration of misoprostol before intrauterine contraceptive insertion following previous insertion failure: a double blind RCT. Hum Reprod. 2015;30(8):1861-1866.
- Lyus R, Lohr P, Prager S. Use of the Mirena LNG-IUS and Paragard CuT380A intrauterine devices in nulliparous women. Contraception. 2010;81(5):367-371.
- Harvey C, Bateson D, Wattimena J, et al. Ease of intrauterine contraceptive device insertion in family planning settings. Aust N Z J Obstet Gynaecol. 2012;52(6):534-539.
- Khalaf M, Amin AF, Sayed Z, et al. A randomized double-blind controlled trial of two different doses of self-administered vaginal misoprostol for successful copper intrauterine device insertion. Middle East Fertil Soc J. 2017;22(4):264-8.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015(7).
- Zapata LB, Jatlaoui TC, Marchbanks PA, et al. Medications to ease intrauterine device insertion: a systematic review. Contraception. 2016;94(6):739-759.
- Abdellah MS, Abbas AM, Hegazy AM, et al. Vaginal misoprostol prior to intrauterine device insertion in women delivered only by elective cesarean section: a randomized double-blind clinical trial. Contraception. 2017;95(6):538-543.
- Sääv I, Aronsson A, Marions L, et al. Cervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trial. Hum Reprod. 2007;22(10):2647-2652.
- Scavuzzi A, Souza AS, Costa AA, et al. Misoprostol prior to inserting an intrauterine device in nulligravidas: a randomized clinical trial. Hum Reprod. 2013;28(8):2118-2125.
- World Health Organization, World Health Organization. Reproductive Health. Medical eligibility criteria for contraceptive use. WHO; 2010.
- Ibrahim ZM, Sayed Ahmed WA. Sublingual misoprostol prior to insertion of a T380A intrauterine device in women with no previous vaginal delivery. Eur J Contracept Reprod Heal Care. 2013;18(4):300-308.
- Khatri A, Kalra N. A comparison of two pain scales in the assessment of dental pain in East Delhi children. ISRN dentistry. 2012;2012.
- Bakas P, Hassiakos D, Liapis A, et al. Misoprostol for cervical ripening before diagnostic hysteroscopy in nulliparous women. Int J Obstet Gynaecol. 2012;116(3):263-264.
- Alanwar A, Naguip S, El-Ghanam A, et al. Two Different Doses of Self-administered Vaginal Misoprostol for Successful Copper Intrauterine Device Insertion in Parous Women Previously Delivered by Cesarean Section-A Double Blinded Randomized Clinical Trial. Gin Pol Med Project. 2022;17(1):1-6.
- Abbas AM, Ali SS, Salem MN, et al. Effect of oral ketoprofen on pain perception during copper IUD insertion among parous women: a randomized double-blind controlled trial. Middle East Fertil. Soc J. 2018;23(4):491-495.
- Oppegaard KS, Nesheim BI, Istre O, et al. Comparison of self‐administered vaginal misoprostol versus placebo for cervical ripening prior to operative hysteroscopy using a sequential trial design. BJOG: An Int J Obstet Gynaecol. 2008;115(5):663-e9.
- Preutthipan S, Herabutya Y. A randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopy. Obstet Gynecol. 1999;94(3):427-430.
- Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96(6):890-894.
- Ngai SW, Chan YM, Liu KL, et al. Oral misoprostol for cervical priming in non-pregnant women. Hum Reprod. 1997;12(11):2373-2375.
- Waddell G, Desindes S, Takser L, et al. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Minim Invasive Gynecol. 2008;15(6):739-744.
- Swenson C, Turok DK, Ward K, et al. Self-administered misoprostol or placebo before intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol.;120(2 Part 1):341-347.
- Arias F. Pharmacology of oxytocin and prostaglandins. Clin Obstet Gynecol. 2000;43(3):455-468.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Mohamed H. Salama1, Shaimaa G. Helmy1, Mohamed E. Elhodiby2, Mohammed A. El-Kadi1 and Maii Nawara1*2Department of Obstetrics & Gynecology, Faculty of Medicine, M.U.S.T University, Egypt
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.