Research - (2022) Volume 0, Issue 0
Effect of progesterone on latent phase prolongation in patients with preterm premature rupture of membranes
El Sayed H*, Abdelhamid S, El Helaly A, Fouad M and Aly LReceived: 26-Aug-2022, Manuscript No. gpmp-22-73044; Editor assigned: 27-Aug-2022, Pre QC No. P-73044; Reviewed: 09-Sep-2022, QC No. Q-73044; Revised: 15-Sep-2022, Manuscript No. R-73044; Published: 29-Sep-2022
Abstract
Objectives: To investigate the effect of progesterone given before 32 week gestation to cases of Preterm Premature Rupture of Membranes (PROM) on prolongation the pregnancy and thereafter reduce neonatal morbidities.
Methods: This study was conducted on 112 pregnant women diagnosed with PPROM and admitted to Ain shams university hospital for standard management. They were divided into two groups: the study group was given weekly IM progesterone and the control group which received a placebo therapy to test the effect of progesterone in prolonging the latency period in such cases. The age of recruited cases was between 18-35 years, carrying Singleton pregnancy, at gestational age between 24-34 weeks. The pregnant women were randomly arranged by closed envelop method according to randomization table into two groups, (placebo group and intramuscular progesterone group), 56women in case (control) group & 56 women in (intramuscular progesterone) group. All patients were followed up until delivery. The primary outcome measure is prolongation of the pregnancy until 34.0 weeks of gestation or documentation of fetal lung maturity at 32.0 to 33.9 weeks.
Results: Respiratory distress syndrome (RDS), neonatal intensive care unit (NICU) admission and neonatal death were statistically non-significant but less frequent among intramuscular group. The present study failed to elucidate an evidence that 17OHP-C is beneficial in women with PROM.
Conclusion: 17-hydroxyprogesterone caproate when given to cases of preterm prelabor rupture of membranes does not show a significant effect of prolongation of latency period in such cases versus placebo.
Keywords
Preterm premature rupture of membranes; Progesterone
Introduction
Prelabor rupture of membranes (PLROM) is disruption of the chorion and amnion before the onset of active labor. It account for a serious perinatal morbidity and mortality. It occurs in 18-20% of pregnancies. Preterm prelabor rupture of membranes (PPLROM) occurs in 1-5% of all pregnancies. It is the underlying cause for nearly 30-40% of all preterm birth.
Preterm delivery is defined as the delivery before the end of the 37th week of gestation and is possibly the single most important health related issue in pregnancy. Latency period is the period from the occurrence of prelabor ROM till start of preterm delivery. Preterm delivery carries the highest risk of morbidity and mortality on the neonates especially in the developing countries nearly 85% [1].
A prolonged latent period in cases of PPROM is not without a risk and is also linked to maternal, fetal and neonatal morbidities especially if infections occurred [2]. The vital organs may be affected as in pulmonary hypoplasia, the heart, enterocolitis, periventricular leukomalacia and intracranial hemorrhages beside the complications of prolonged oligohydramnios with the development of adhesion bands that may lead to amputations of limbs [3-5].
Now it is possible to screen for patients at a risk of developing preterm labor and PPROM by the serial transvaginal measurement of cervical length and cerivicovaginal fetal fibronectin which is the glue that connect the fetal chorion to maternal decidua and rise in the level of it is denoting fetal membranes disruption [6-8]. Unfortunately, uptill now there is no sufficient therapeutic option to suppress or delay birth.
In cases of PPROM, management options include bed rest, oral fluids, no coitus or PV examination, prophylactic antibiotics, corticosteroids to enhance fetal lung maturity [9], tocolytics [10], cervical cerclage [11] and most notably progesterone [12-14].
The mechanism of action of Progesterone includes a strong effect in suppressing premature birth [15]. However there is a dilemma regarding if it is should be used in the clinical practice and if used what is the best route and the effective dose of such medication [1,16].
Progestins as progesterone and 17- hydroxyprogesterone caproate (17P) were used effectively to suppress preterm labor specially in patients with short cerviix [17,18], and those who responded well to tocolysis [19,20]. Still, the role of 17P in PPROM is not well studied. Several actions may be proposed in prolonging the latent phase of prelabor ROM such as inhibiting the inflammatory substrates, depression of the production of gap junction proteins which are the contractions associated proteins and have a major role in the progression of preterm birth [21].
The present study was undertaken to test the hypothesis that 17P given to mothers with PROM before 32 weeks of gestation will prolong the pregnancy and thereby reduce neonatal morbidity.
Patient and Methods
Type of the Study: Randomized controlled interventional prospective study.
Study Settings: This clinical trial was conducted at Ain-Shams University Maternity Hospital, in the period from January till December 2020.
Study Population: The patients were recruited from women admitted from obstetric clinic or from emergency room and from patients following up in outpatient clinic 112 patients will be recruited to this study.
Sample size justification: Depending on Combs et al. [22] who found that Mean ± SD of GA at delivery in IM progesterone and placebo groups 30.0 ± 4.0 and 28.0 ± 3.0 days respectively, and assuming the power= 0.80 and α=0.05, and by using PASS 11th release the minimal sample size for an equal size a controlled clinical trial is 51 in each group.
A drop out of 10% was included to avoid the dropped out patients.
Inclusion criteria
• Singleton pregnancy.
• Gestational age between 24 and 34 weeks.
• They are admitted for Preterm premature rupture of membranes and received prophylactic antibiotics.
Exclusion criteria
1. Medical or obstetric conditions that could put them at risk for uterine atony and postpartum hemorrhage and infection, such as:
• Emergency Caesarean section
• Chorioamnionitis
• Placenta previa
• Multiple gestation
• Preeclampsia.
Macrosomia.
2. Non reassuring fetal status or fetal distress
3. Presence of fetal anomalies incompatable with life
4. Woman with antepartum hemorrhage
5. Diagnosis of Established preterm labor
The included patients were randomized using sealed opaque envelope method into one of two groups:
Group I (study group): In which 17-hydroxyprogesterone caproate (17P) (250 mg in castor oil, 1 mL total volume, intramuscular injection weekly) administered in 56 patients.
Group II (control group): In which an identical-appearing placebo (1 mL castor oil only) administered another 56 patients.
Randomization and Blinding
• Included patients were randomized into one of the study groups. Randomization was performed using a computer-generated randomization system (Microsoft office excel, 2007).
• A plan of management was sealed in closed envelops, numbered according to the randomization tables. Packing, sealing and numbering were all performed by 2 independent doctors other than the investigator.
• Neither the investigator nor the residents following up the patient were aware which patient had received17-hydroxyprogesterone caproate (17P) therapy and which hadn’t (double-blinding). Randomization coding tables were hidden from the investigator till the end of the study.
Ethics: Informed written consent was obtained from all participants before recruitment in the study, and after explaining the purpose and procedures of the study and possible risks.
Methodology
After taking informed written consent, the recruited patients were subjected to the following:
1. Detailed history and full Examination of the patients (General and abdominal)
2. Pelvic Examination only by sterile cusco speculum to exclude cord prolapse , bloody liquor and cervical dilatation and effacement
3. Non stress test to ensure reassuring fetal well being
4. Ultrasound examination to:-
Assess fetal viability.
Amniotic fluid index.
Determine gestational age.
Exclude major anomalies.
Placental location.
5. Baseline laboratory investigations: CBC, PT, PTT, Liver and renal function tests
PPROM was established by vaginal examination using sterile Cusco speculum with trickling of liquor from the cervix, pooling of liquor in the posterior fornix or presence of meconium in the vagina which was performed by a single team of physicians.
Sonography was performed with documentation of the estimated gestational age and assessing the Amniotic fluid index by 4 pocket method.
Women were divided into two groups; the intervention group which received 17P IM weekly injections till completed 36 completed weeks and the control group which received placebo (1ml castor oil). All patients received the standard management of PPROM in the form of hospital admission, prophylactic antibiotics and betamethasone to enhance fetal lung maturity. Tocolytics were not given. Fetal surveillance was performed on regular basis (daily Fetal Non-Stress Test and bi-weekly biophysical profile). Signs of chorioamnionitis were watched.
Primary outcomes
Prolongation of the pregnancy until a favorable gestational age, which we defined as either 34.0 weeks of gestation or documentation of fetal lung maturity at 32.0 to 33.9 weeks.
The investigators agreed that continuation of pregnancy beyond these time points was not indicated.
2ry outcomes
1. Latency (interval from randomization to delivery).
2. Composite neonatal morbidity, which will be defined per Mercer et al. [23] as one or more of: stillbirth, neonatal death, infant death before hospital discharge, respiratory distress syndrome (RDS), intracranial hemorrhage (ICH) grade 3 or 4, necrotizing enterocolitis (NEC) stage 2 or 3, culture-proven neonatal sepsis within 72 h of birth. In addition to the components defined by Mercer et al., [23] our definition of composite neonatal morbidity included periventricular leukomalacia (characteristic lesions in the subcortical white matter seen on cerebral imaging studies within 96 h of birth). The occurrence of chorioamnionitis, and puerperal metritis.
Results
Fig. 1. Presents the flow chart of studied cases and Tab. 1. Shows that No significant difference between the studied groups regarding baseline characteristics. Tab. 2. And Fig. 2. Shows that there is no significant difference between the studied groups regarding causes of termination; contractions, chorioamnionitis and fetal distress. Tab. 3. Shows that no significant difference between the studied groups regarding enrollment and gestational age at time of delivery. Latency period was non-significantly higher among study group than among control group. Fig. 3. Show that no significant difference between the studied groups regarding rate of delivery. Tab. 4. Shows that no significant difference between the studied groups regarding gestational age at delivery. Tab. 5. Shows that no significant difference between the studied groups regarding neonatal condition. Tab. 6. and Fig. 4. Shows that neonatal complications were non-significantly less frequent among study group than among control group. Death was non-significantly less frequent among study group than among control group. Fig. 5. Indicates the death among the studied groups.
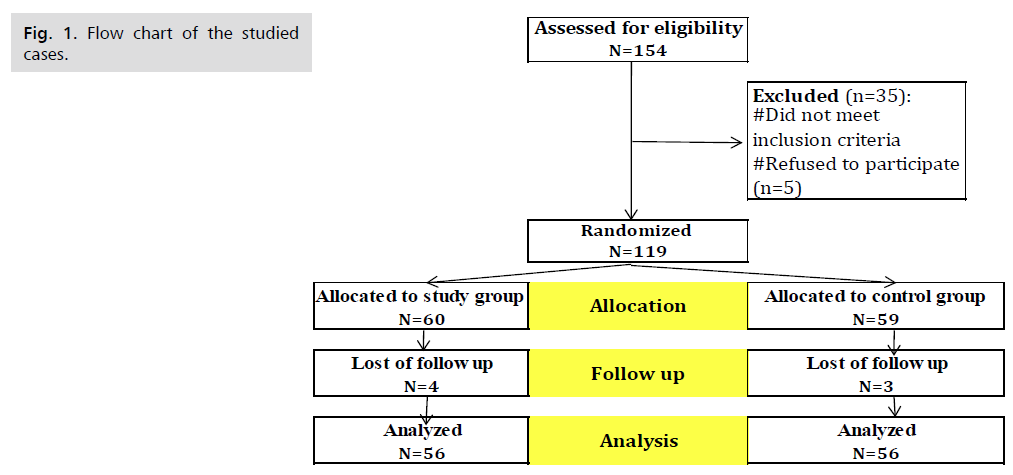
Fig 1. Flow chart of the studied cases.
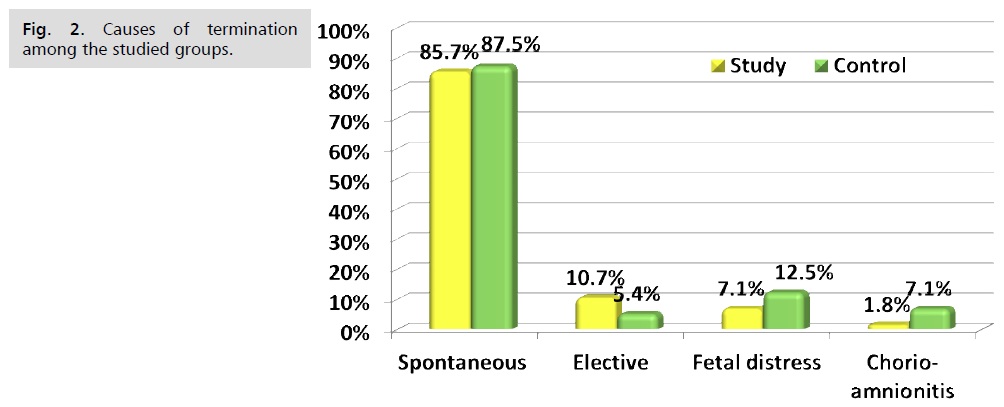
Fig 2. Causes of termination among the studied groups.
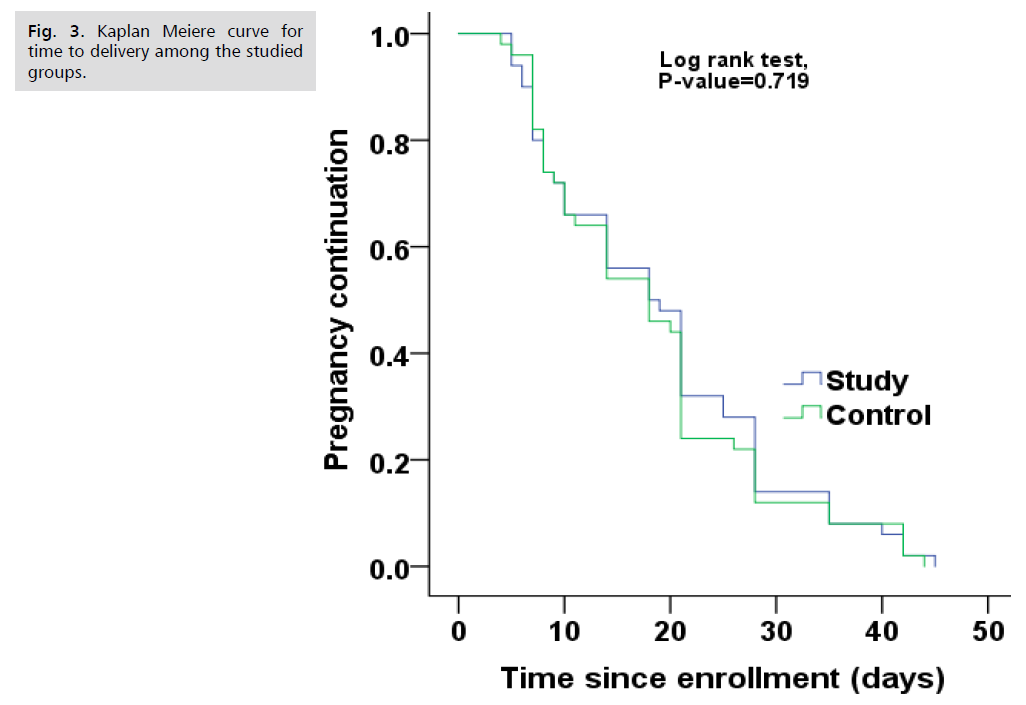
Fig 3. Kaplan Meiere curve for time to delivery among the studied groups.
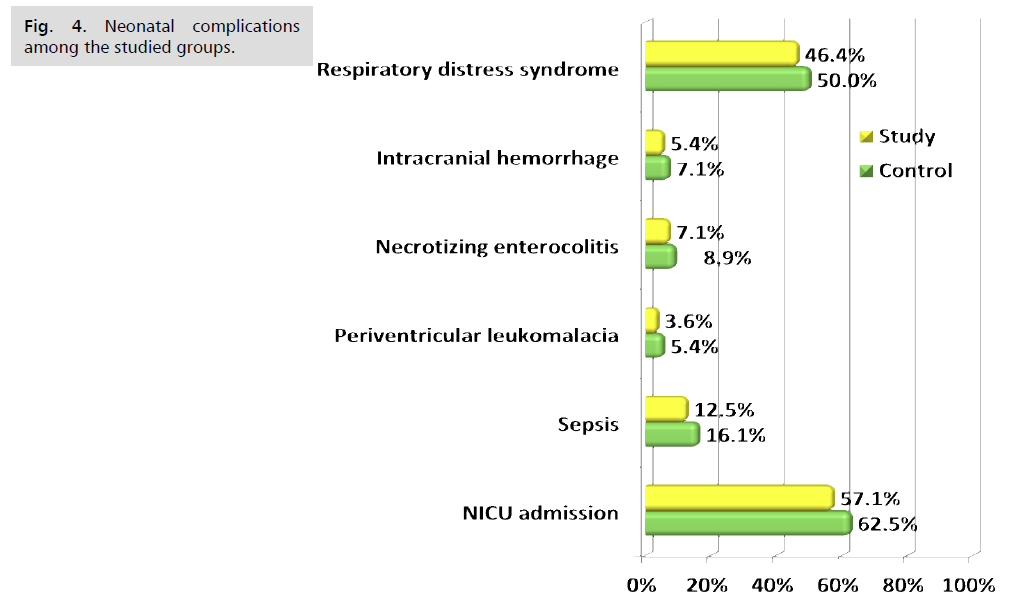
Fig 4. Neonatal complications among the studied groups.
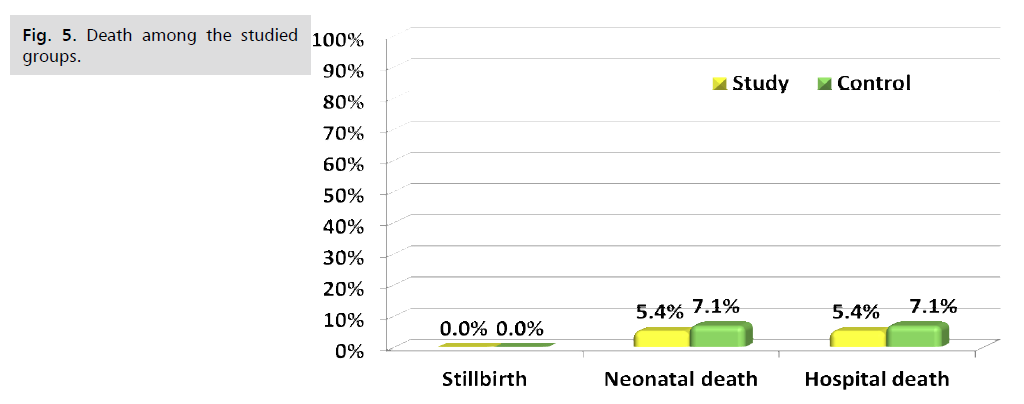
Fig 5. Death among the studied groups.
| Items | Measure | Study (N=56) | Control (N=56) | P-value |
|---|---|---|---|---|
| Age (years) | Mean±SD | 28.2±4.6 | 29.3±3.9 | ^0.293 |
| Range | 19.0–35.0 | 20.0–35.0 | ||
| BMI (kg/m2) | Mean±SD | 30.6±3.2 | 31.0±3.3 | ^0.448 |
| Range | 24.5–36.0 | 24.9–36.2 | ||
| Parity (n, %) | Primi | 17 (30.4%) | 15 (26.8%) | #0.676 |
| Multi | 39 (69.6%) | 41 (73.2%) | ||
| Hemoglobin (gm/dL) | Mean±SD | 11.4±0.9 | 11.3±0.8 | ^0.876 |
| Range | 9.6–13.8 | 9.9–13.4 | ||
| TLC (x103/mL) | Mean±SD | 7.3±0.8 | 7.5±0.8 | ^0.208 |
| Range | 5.9–9.8 | 5.6–9.2 | ||
| CRP | Positive | 0 (0.0%) | 0 (0.0%) | Not applicable |
| Negative (<10 mg/L) | 56 (100.0%) | 56 (100.0%) | ||
| History of previous abortion | 8 (14.3%) | 6 (10.7%) | #0.568 | |
| History of previous preterm labor | 3 (5.4%) | 2 (3.6%) | §0.999 | |
Tab. 1. Baseline characteristics among the studied groups.
| Findings | Study (N=56) | Control (N=56) | P-value | RR (95% CI) |
|---|---|---|---|---|
| Spontaneous | 48 (85.7%) | 49 (87.5%) | #0.781 | 0.98 (0.85–1.13) |
| Elective | 6 (10.7%) | 3 (5.4%) | §0.489 | 2.00 (0.53–7.60) |
| Fetal distress | 4 (7.1%) | 7 (12.5%) | #0.341 | 0.57 (0.18–1.84) |
| Chorioamnionitis | 1 (1.8%) | 4 (7.1%) | §0.364 | 0.25 (0.03–2.17) |
| labs | N=1 | N=4 | Mean±SE 95% CI |
|
| CRP (mg/(L) | 16.3 | 16.9±1.2 | ^0.697 | -0.6±1.3 -4.6– 3.5 |
| TLC (x103/mL) | 24.0 | 15.0±6.0 | ^0.685 | -3.0±6.7 -24.4–18.3 |
Tab. 2. Causes of termination among the studied groups.
| Time | Measure | Study (N=56) | Control (N=56) | ^P-value | Mean±SE 95% CI |
|---|---|---|---|---|---|
| Enrollment (week) | Mean±SD | 28.2±2.7 | 28.4±2.8 | 0.701 | -0.2±0.5 |
| Range | 24.1–34.0 | 24.3–34.0 | -1.2–0.8 | ||
| Delivery (week) | Mean±SD | 31.2±3.4 | 31.6±3.6 | 0.495 | -0.4±0.7 |
| Range | 25.1–36.0 | 25.1–36.0 | -1.8–0.9 | ||
| Latency period (days) | Mean±SD | 20.5±12.0 | 17.7±11.3 | 0.215 | 2.8±2.2 |
| Range | 4.0–44.0 | 5.0–45.0 | -1.6–7.1 |
Tab. 3. Enrollment, gestational age at time of delivery and latency period among the studied groups.
| Time (weeks) | Study (N=56) | Control (N=56) | ^P-value |
|---|---|---|---|
| <28.0 | 13 (23.2%) | 14 (25.0%) | #0.203 |
| 28.0−32 | 17 (30.4%) | 8 (14.3%) | |
| 32.0−34 | 9 (16.1%) | 14 (25.0%) | |
| ≥ 34.0 | 17 (30.4%) | 20 (35.7%) |
Tab. 4. Gestational age at delivery among the studied groups.
| Time | Measure | Study (N=56) | Control (N=56) | ^P-value | Mean±SE 95% CI |
|---|---|---|---|---|---|
| Birth weight (kg) | Mean±SD | 1.9±0.9 | 1.7±0.9 | ^ 0.352 | 0.2±0.2 |
| Range | 0.5–3.1 | 0.6–3.0 | -0.2–0.5 | ||
| APGAR (first minute) | Mean±SD | 5.6±1.3 | 5.6±1.2 | ^ 0.879 | 0.0±0.2 |
| Range | 4.0–8.0 | 4.0–8.0 | -0.4–0.5 | ||
| APGAR (fifth minute) | Mean±SD | 6.4±1.4 | 6.3±1.2 | ^ 0.560 | 0.1±0.2 |
| Range | 4.0–9.0 | 4.0–9.0 | -0.3–0.6 | ||
| Blood PH | Mean±SD | 7.3±0.1 | 7.3±0.1 | ^ 0.516 | -0.01±0.02 |
| Range | 7.1–7.5 | 7.2–7.6 | -0.04–0.02 | ||
| Labs | RR (95% CI) | ||||
| WBC (x103/mL) | ≥25.0 | 7 (12.5%) | 9 (16.1%) | #0.589 | 0.78 |
| <25.0 | 49 (87.5%) | 47 (83.9%) | (0.31–1.94 | ||
| CRP (mg/L) | ≥10.0 | 7 (12.5%) | 9 (16.1%) | #0.589 | 0.78 (0.31–1.94 |
| <10.0 | 49 (87.5%) | 47 (83.9%) | |||
Tab. 5. Neonatal condition among the studied groups.
| Complications | Study (N=56) | Control (N=56) | P-value | RR (95% CI) |
|---|---|---|---|---|
| Neonatal | ||||
| Respiratory distress syndrome | 26 (46.4%) | 28 (50.0%) | #0.705 | 0.93 (0.63–1.36) |
| Intracranial hemorrhage | 3 (5.4%) | 4 (7.1%) | §0.999 | 0.75 (0.18–3.20) |
| Necrotizing enterocolitis | 4 (7.1%) | 5 (8.9%) | §0.999 | 0.80 (0.23–2.82) |
| Periventricular leukomalacia | 2 (3.6%) | 3 (5.4%) | §0.999 | 0.67 (0.12–3.84) |
| Sepsis (culture proven) | 7 (12.5%) | 9 (16.1%) | #0.589 | 0.78 (0.31–1.94) |
| NICU admission | 32 (57.1%) | 35 (62.5%) | #0.563 | 0.91 (0.67–1.24) |
| NICU admission duration (days) N=32,35 |
3.25±1.19 | 3.09±1.20 | ^0.576 | 0.2±0.3 |
| 2.00–5.00 | 1.00–6.00 | -0.4–0.7 | ||
| Mortality | ||||
| Stillbirth | 0 (0.0%) | 0 (0.0%) | -- | -- |
| Neonatal death | 3 (5.4%) | 4 (7.1%) | §0.999 | 0.75 (0.18–3.20) |
| Hospital death | 3 (5.4%) | 4 (7.1%) | §0.999 | 0.75 (0.18–3.20) |
Tab. 6. Neonatal complications and mortality among the studied groups.
Discussion
PPROM has many adverse effects on many vital organs of the fetus and neonate. It can cause pulmonary hypoplasia, skeletal deformities due to affection of fetal movements, also, the development of intraamniotic adhesion bands which may lead to amputations of limbs. The cardiovascular and central nervous systems are markedly affected in preterm babies [1,3-5,24]. These complications become more frequent and severe in maternal morbidities as chorioamnionitis and sepsis [25].
Management of PPROM is usually by expectant management including hospitalization, best rest, fluids, antibiotics, steroids and magnesium sulphate for neuroprotection. The second option is or delivery when the risks of expectant treatment outweigh the benefit [24].
The present study was a prospective, randomized controlled trial to evaluate the effect of intramuscular injection of 17-hydroxyprogesterone corporate (17-OHPC) on latency of pregnancy in patients with PPROM, aiming to prevent the perinatal morbidities and mortality. Eligible 112 patients (according to our inclusion criteria which are patient with PPROM confirmed on clinical examination were randomly allocated to one of two treatment arms in a double blind manner by computer generated system. A total number of 154 patients were recruited and eligible to participate in the study. 30 patient excluded from study not meet inclusion criteria and 5 patient refused to participate. Among them 119 consented to participate. They were randomized to placebo group and study group. The first group consists of 60 patients with 4 lost in follow up and the second group 59 patient with 3 lost in follow up
In the study group, progesterone intramuscular (Cidolut depot) (250 mg per week) were administered until delivery or completion of the 36th gestational week and was compared with placebo effect (intramuscular castor oil) in control group. The latent phase and maternal and neonatal outcome variables were compared between the two groups. The two groups were almost identical in the confounding factors, as regards demographic characteristics: Age, parity, BMI (kg/m2), Enrollment (weeks) (p values= 0.293, 0.676, 0.448, 0.701) respectively not to affect the study results and interpretation. The mean latent phase was 20.5 ± 12 days in the intervention group vs.17.7 ± 11.3 days in the control group, which was statistically non-significant in the intervention group (P=0.215).
The study results showed that it is no statistically significant difference between the two groups regarding number of neonatal RDS, NICU admission and neonatal death but lower percentage among study group. NICU stay was statistically insignificant between 2 groups.
There is prolongation in the mean GA in (Days) in the study group compared to control group but with non-statistically significance (P value =0.215, CI = -1.6 -7.1).
This result is in agreement with [26] who had performed a similar study on 1228 women and also reported that 17P was not effective in prolonging the latency period. Also the drug didn’t increase the maternal or neonatal morbidities or mortalities.
Similar results was reported by Combs et al. [22] and Quist-Nelson et al. [27] who investigated 17P in singleton gestations with PPROM (n=545 participants). 17-OHPC intake was not associated with a significant increase of the latency period. They also concluded that progesterone did not increase morbidity or mortality in the mother or the child.
Abdali et al. [28] used progesterone suppositories (400 mg per night) in his study on 120 patients till 34th gestational week and were compared with placebo effect in control group. The latent phase and maternal and neonatal outcome variables were compared between the two groups.
Mirzaei et al. [29] also performed a clinical study on patients with PPROM. 171 PPROM patients were selected, group 1 (57 patients) using 17OHP 250 mg/week, group 2 (57 patients) using 400 mg progesterone suppositories/day, and group 3 (57 patients) using 17OHP 250 mg/day. 102 patients) did not take the drug. The mean latency from rupture of membranes to delivery was 15/5 days in the first group, 15/2 days in progesterone recipients, and 11/5 days in unmedicated patients. This difference was statistically significant, which contradicts our study.
In a similar study done by Da Fonseca et al. [30] on 142 women using progesterone (100 mg suppository/day) with placebo. Progesterone significantly reduced the rate of premature birth. This contradicts our studies on the effective role of progesterone in prolonging latency, route of administration, dose, and large sample sizes.
Conclusion and Recommendations
Weekly intramuscular 17-hydroxyprogesterone caproate does not prolong the latency in PPROM versus placebo. Further studies with larger number of patients are needed to prove or refute our results.
Authors Contribution
(A) Study Design · (B) Data Collection. (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- Norwitz ER and Caughey AB.Progesterone supplementation and the prevention of preterm birth. Rev Obstet Gynecol. 2011;4:60.
- Greulich B and Tarrant B. The latent phase of labor: diagnosis and management. J Midwifery Women Health. 2007;52:190-8.
- Jones M. Effect of preterm birth on airway function and lung growth. Paediatr Respir Rev. 2009;10:9-11.
- Spittle AJ, Treyvaud K, Doyle LW, et al. Early emergence of behavior and socialemotional problems in very preterm infants. J Am Acad Child Psychiatry. 2009;48:909-18.
- Ibrahimou B, Anozie C, de la Cruz C, et al. Previous Preterm Birth and Current Maternal Complications as a Risk Factor of Subsequent Stillbirth. Adv Epidemiol. 2015;2015:819146.
- Khooshideh M, Radi V, Hosseini R, et al. The accuracy of placental alpha-microglobuline-1 test in diagnosis of premature rupture of the membranes. Iran J Reprod Med. 2015;13(6): 355.
- Gezer C, Ekin A, Golbasi C, et al. Use of urea and creatinine levels in vaginal fluid for the diagnosis of preterm premature rupture of membranes and delivery interval after membrane rupture. J Matern Fetal Neonatal Med. 2016;30:772-8.
- Lee SM, Park KH, Jung EY, et al. Frequency and clinical significance of short cervix in patients with preterm premature rupture of membranes. PloS One 2017;12:e0174657.
- Brookfield KF, El-Sayed YY, Chao L, Berger V, Naqvi M, Butwick AJ. Antenatal corticosteroids for preterm premature rupture of membranes: single or repeat course? Am J Perinatol. 2015;32:537-44.
- Mackeen AD, Seibel‐Seamon J, Muhammad J, et al. Tocolytics for preterm premature rupture of membranes. Cochrane Database Syst Rev. 2011;10:CD007062.
- To MS, Alfirevic Z, Heath VC, et al. Cervical cerclage for prevention of preterm delivery in woman with short cervix: randomised controlled trial. Lancet. 2004;363:1849-53.
- Lim AC, Bloemenkamp KW, Boer K, et al. Progesterone for the prevention of preterm birth in women with multiple pregnancies: the AMPHIA trial. BMC Pregnancy Childbirth. 2007;7:7.
- How HY and Sibai BM. Progesterone for the prevention of preterm birth: indications, when to initiate, efficacy and safety. Ther Clin Risk Manag. 2009;5:55-64.
- Kumar P and Magon N. Hormones in pregnancy. Niger Med J. 2012;53:179.
- Luo G, Abrahams VM, Tadesse S, et al. Progesterone inhibits basal and TNF-αinduced apoptosis in fetal membranes: a novel mechanism to explain progesterone-mediated prevention of preterm birth. Reprod Sci. 2010;17:532-9.
- Briery CM, Veillon EW, Klauser CK, et al. Women with preterm premature rupture of the membranes do not benefit from weekly progesterone. Am J Obstet Gynecol. 2011;204:54-5.
- DeFranco EA, O’Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007; 30:697-705.
- Fonseca EB, Celik E, Parra M, et al. Progesterone and the risk of preterm birth among women with a short cervix. New Engl J Med. 2007;357:462-469.
- Facchinetti F, Paganelli S, Comitini G, et al. Cervical length changes during preterm cervical ripening: effects of 17-alphahydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196:453-454.
- Borna S and Sahabi N. Progesterone for maintenance tocolytic therapy after threatened preterm labour: A randomized controlled trial. Aust NZ J Obstet Gynaecol. 2008;48:58-63.
- Hirota Y, Cha J, Dey SK. Prematurity and the puzzle of progesterone resistance. Nature Med . 2010;16:529-531.
- Combs CA, McCune M, Clark R and Fishman A: Aggressive tocolysis does not prolong pregnancy or reduce neonatal morbidity after preterm premature rupture of the membranes. Am J Obstet Gynecol. 2004;190:1723-31.
- Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101:178-193.
- American college of Obstetricians and gynecologists: Prelabor Rupture of Membranes, Practice Bulletin No. 188: Obstet Gynecol. 2018;131:e1.
- Linehan LA, Walsh J, Morris A, et al. Neonatal and maternal outcomes following midtrimester preterm premature rupture of the membranes: a retrospective cohort study. BMC Pregnancy Childbirth. 2016;16:25.
- Wilson E, Maier RF, Norman M, et al. Admission hypothermia in very preterm infants and neonatal mortality and morbidity. J Pediatr. 2016;175:61-7.
- Quist-Nelson J, de Ruigh AA, Seidler AL, et al. Preterm Premature Rupture of Membranes Meta-analysis (PPROMM) Collaboration. Immediate Delivery Compared With Expectant Management in Late Preterm Prelabor Rupture of Membranes: An Individual Participant Data Meta-analysis. Obstet Gynecol. 2018;131:269-279.
- Abdali F, Taghavi S, Vazifekhah S, et al. Effect of progesterone on latent phase prolongation in patients with preterm premature rupture of membranes. Acta Medica Iranica. 2017:772-8.
- Mirzaei F, Moradi P. Effects of progesterone on latency period in patients with preterm premature rupture of membranes during 24-34 weeks of pregnancy. J Kerman Univ Medical Sci. 2015;22(3):240-8.
- Da Fonseca EB, Bittar RE, Carvalho MH, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419-424.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar , Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
El Sayed H*, Abdelhamid S, El Helaly A, Fouad M and Aly LCopyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.