Research - (2024) Volume 19, Issue 4
Cytokines and hormones in polycystic ovary syndrome: a biochemical study in Iraqi women
Nadia Kamile Al-Mashta1*, Suzan Hammed Uraibi1, Athraa Imad Habeeb2, Shaima M. Al Sabty1,3 and Saif M. Hassan4Received: 02-Dec-2024, Manuscript No. gpmp-24-155969; Editor assigned: 03-Dec-2024, Pre QC No. P-155969; Reviewed: 16-Dec-2024, QC No. Q-155969; Revised: 23-Dec-2024, Manuscript No. R-155969; Published: 30-Dec-2024
Abstract
Background: In Polycystic Ovary Syndrome (PCOS), sex hormones exert a regulatory influence over ovarian follicular development and a number of other functions within the body. It has been postulated that cytokines may operate as putative local regulators in PCOS.
Methodology: The prospective research groups were divided into six categories according to Body Mass Index (BMI), with a total of 150 women enrolled in the study. The aim was to investigate the link between sex hormones and inflammation markers in women with different body types and without PCOS.
Results: The results of the study indicated a notable decline (p<0.05) in hormone levels in the non-PCOS groups when compared to the PCOS groups. The lowest levels of TNF-α, IL-6 and IL-8 were observed in the non-PCOS groups. The highest levels were observed in women with PCOS.
Conclusion: Sex hormone levels get greater in a weight-dependent manner and in the presence of PCOS scenario.
Keywords
BMI; Cytokine; Sex hormones; Polycystic ovary syndrome
Introduction
Obesity is linked to a condition called polycystic ovary syndrome (PCOS). PCOS causes irregular or heavy periods. Common features include higher testosterone and insulin levels, and lower levels of a hormone called sex hormone-binding globulin. This explains why there is a similarity in the symptoms of these conditions [1]. Chronic anovulation, hyperandrogenism and polycystic in ovaries are the most frequent endocrine condition in women between the ages of 18 and 44 [2]. It has been demonstrated that PCOS is a chronic low-grade inflammatory condition [3]. In vitro studies indicate that pro-inflammatory stimuli may induce the upregulation of steroidogenic enzymes in theca cells of the ovary, thereby contributing to the production of androgens and resulting in hyperandrogenism. This concept suggests that inflammation may be a direct cause of hyperandrogenism in PCOS [4].
In humans, adipocyte TNF-α levels are linked to obesity, but there is significant heterogeneity in expression, suggesting additional mechanisms are responsible. There is a correlation between obesity and insulin resistance, as well as the elevation of TNF-α in adipose tissue and skeletal muscle. This is because TNF-α is responsible for insulin resistance through the autocrine-paracrine pathway [5]. In obese adipocytes, the production of IL-6 and IL-8 is increased, and this may potentially influence the action of insulin either locally or circulatingly. TNF-α has been studied a lot in relation to insulin resistance, but only a few studies have involved human subjects. None of these have looked at how cytokine expression affects insulin resistance in obese people [6].
Method
Study groups
The prospective study groups consist of 6 groups according to BMI, with a total of 150 women. Each group is as follows:
- Group 1: normal-weight non‐PCOS women with BMI (20.0-23.0 kg/m2)
- Group 2: overweight non‐PCOS women with BMI (28.0–32.0 kg/m2)
- Group 3: obese non‐ PCOS women with BMI (>32.0 kg/m2)
- Group 4: Normal-weight PCOS women with BMI (20.0-23.0 kg/m2)
- Group 5: Overweight PCOS women with BMI (28.0-32.0 kg/m2)
- Group 6: Obese PCOS women with BMI (>32.0 kg/m2)
Biochemical measurement
The clinical characteristics of the women, including the presence of acne and hirsutism, were evaluated. Additionally, the subjects' metabolic metrics, including their body mass index, insulin resistance as evaluated using the homeostatic model, serum insulin, and glucose concentration, were assessed. The following hormonal parameters were the focus of the assessment: Follicle-Stimulating Hormone (FSH), free testosterone, Dehydroepiandrosterone Sulfate (DHEA-S), and Luteinizing Hormone (LH). In addition to the measurement of serum human IL‐6, TNF‐α, and IL‐8 concentrations, cortisol levels and estradiol were also measured in order to provide a comprehensive analysis of the endocrine and inflammatory profile.
Statistical analysis
A univariate linear regression model was employed to examine the relationship between BMI and various biomarkers. The objective of this analysis was to determine whether a connection exists between BMI and these markers. Additionally, we analysed how age influences biomarker levels in the body. To this end, a Student's t-test was conducted to compare groups across different time points, and a linear model was utilized to assess the impact of BMI on biomarker levels.
RESULTS
The effect of obesity on sex hormones and polycystic ovary syndrome
The findings of the present study demonstrated a substantial decrease (p<0.05) in the levels of sex hormones in women diagnosed with non-polycystic ovary syndrome (non-PCOS) when compared to those with PCOS. A statistically significant difference was observed between the groups without PCOS; however, obese women exhibited the highest levels. Serum levels of LH, FSH, testosterone, and DHEA-S were found to be two to three times higher in women with PCOS compared to those without the condition. The mean differences in biochemical data are presented in Tab. 1. and Fig. 1.
| Groups/Hormones | LH (µIU/mL) Means ± SD |
FSH (µIU/mL) Means ± SD | Testosterone (nmol/L) Means ± SD |
DHEA-s (nmol/L) Means ± SD |
|---|---|---|---|---|
| (G1) | 6.39 ± 2.21 | 5.56 ± 2.08 | 0.68 ± 0.13 | 123.21 ± 29.63 |
| (G2) | 6.49 ± 1.94 | 5.96 ± 2.98 | 0.87 ± 0.31 | 148.40 ± 49.52 |
| (G3) | 9.11 ± 2.82 | 8.19 ± 2.77 | 1.72 ± .53 | 243.06 ± 83.71 |
| (G4) | 12.54 ± 4.83 | 10.14 ± 3.84 | 2.51 ± 0.88 | 347.13 ± 90.56 |
| (G5) | 16.54 ± 3.8 | 10.14 ± 3.88 | 3.21 ± 0.28 | 397.13 ± 62.51 |
| (G6) | 19.54 ± 5.53 | 14.12 ± 2.59 | 3.89 ± 0.31 | 447.13 ± 62.46 |
| P value | * | * | * | * |
| Significant (α=0.05) | Yes | Yes | Yes | Yes |
Tab. 1. Biochemical variables in studied groups. G1: normal-weight non-PCOS women, G2: overweight non-PCOS women, G3: obese non-PCOS women, G4: normal-weight PCOS women, G5: overweight PCOS women. G6: Obese PCOS women. LH (Luteinizing Hormone), FSH (Follicle Stimulating Hormone), and DHEA-S (Dehydroepiandrosterone sulfate). The P-value was less than 0.05, indicating a statistically significant result.
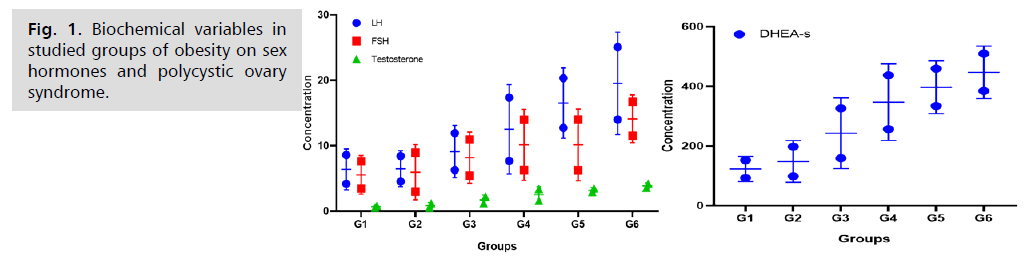
Fig. 1.Biochemical variables in studied groups of obesity on sex hormones and polycystic ovary syndrome.
The present investigation observed the serum levels of TNF-α, IL-6 and IL-8 in 150 women, including those with normal weight, overweight and obese, as well as patients with and without PCOS. The results are reported in Tab. 2. and Fig. 2. It was observed that the lowest values for TNF-α, IL-6 and IL-8 were reported in participants without PCOS. The highest values were observed in PCOS women. Among non-PCOS women, there is significant difference (p<0.05) when compared between. However, there is significant difference between non-PCOS and PCOS women.
| Groups/Hormones | TNF‐α (pg/mL) Means ± SD |
IL‐6 (pg/mL) Means ± SD |
IL‐8 (pg/mL) Means ± SD |
|---|---|---|---|
| (G1) | 127.8 ± 9.5 | 22.6 ± 2.3 | 16.1 ± 4.2 |
| (G2) | 186.8 ± 35.8 | 241.2 ± 11.5 | 347.2 ± 51.4 |
| (G3) | 273.7 ± 85.1 | 315.0 ± 4.0 | 437.1 ± 101.4 |
| (G4) | 652.1 ± 95.2 | 532.1 ± 28.8 | 576.3 ± 109.4 |
| (G5) | 857.4 ± 112.9 | 642.2 ± 11.0 | 682.9 ± 117.0 |
| (G6) | 941.6 ± 105.68 | 1089.9 ± 98.2 | 975.3 ± 111.6 |
| P value | * | * | * |
| Significant (α=0.05) | Yes | Yes | Yes |
Tab. 2. Biochemical variables in studied groups TNF-α, IL-6 and IL-8 levels and obesity-related polycystic ovary syndrome.
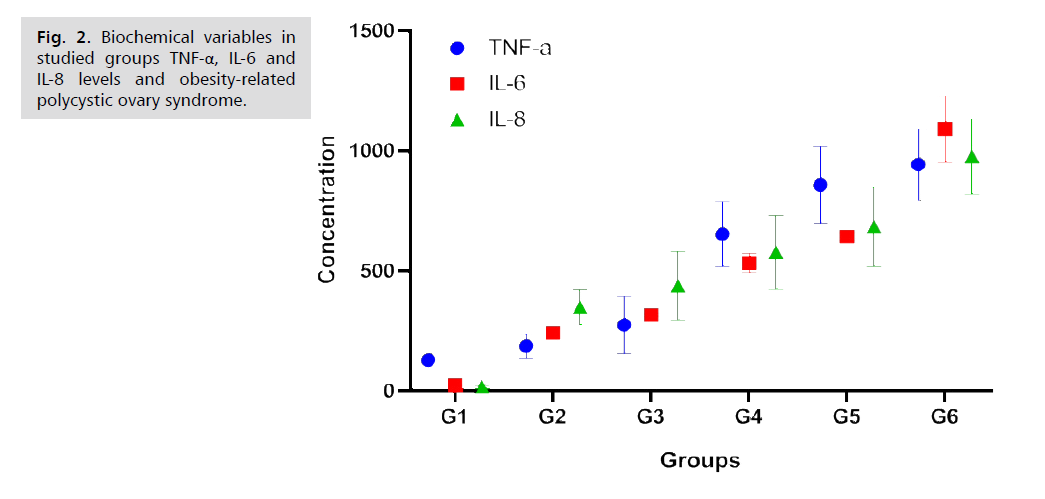
Fig. 2.Biochemical variables in studied groups TNF-α, IL-6 and IL-8 levels and obesity-related polycystic ovary syndrome.
Correlations between biomarkers and BMI
Hormones were correlated with percent body fat (r=0.95, p<0.0001) in women with and without PCOS compared with women with average BMI. The immunological markers levels were measured in the same BMI categories. Individuals with a high body fat percentage and a high BMI tended to have higher levels of TNF-α, IL-6 and IL-8. These alterations were statistically significant, nevertheless, and there was a substantial correlation between rising obesity and immunity marker.
Discussion
The results of our investigation suggested that the levels of hormones were higher in women with PCOS than in those without the disorder. This shows that there may be a relationship between the two. Furthermore, the findings indicated that obese women exhibited higher levels of hormones than non-obese women, which could indicate a correlation between hormonal levels and adipose tissue. Additionally, the data show that there may be a relationship between the disease itself and these hormonal and metabolic parameters.
In Polycystic Ovarian Syndrome (PCOS), the hypothalamic-pituitary-ovarian axis is disturbed, resulting in an increased ratio of Luteinizing Hormone (LH) to Follicle-Stimulating Hormone (FSH) and enhanced pulse frequency of gonadotropin-releasing hormone (GnRH) [7,8]. The primary cause of the symptoms of clinical hyperandrogenism is an excess of androgen produced by ovarian theca cells due to a high LH/FSH ratio. Low levels of serum Sex Hormone-Binding Globulin (SHBG), abnormal gonadotropin (LH and FSH), high levels of adrenal and ovarian androgen and estrogen, and occasionally raised blood insulin are characteristics of the endocrine profile of women with PCOS [9].
In women with polycystic ovarian syndrome (PCOS), the high Luteinizing Hormone (LH) to Follicle-Stimulating Hormone (FSH) ratio results in the failure of ovulation. The major treatments for PCOS are lifestyle adjustments, primarily weight loss, in conjunction with medications [10]. It has been found that between 50 and 90% of women with PCOS exhibit high androgen levels, principally testosterone and DHEAs, depending on the androgens under evaluation and the methods applied. Nevertheless, considerable inter-individual heterogeneity is seen, with some participants having complete hormonal androgen levels [11,12].
The current study found a relationship between Iraqi women with PCOS and those without PCOS in terms of immunological markers levels in BMI. Our investigation has demonstrated a substantial positive connection between these indicators and fat mass in PCOS and non-PCOS participants. The literature on raised cytokines marker levels in female infertility and obese PCOS is scarce.
TNF-α is produced by adipocytes and is increased in the adipose tissue of obese subject. This may play a key role in obesity-related insulin resistance [13]. Despite the proven association between adipocyte TNF-α concentration and obesity, considerable interindividual heterogeneity has been found in humans, indicating that additional variables may influence TNF-α expression [14]. A cytokine that is secreted by a variety of cell types, including adipocytes and adipose stromal cells. It has been demonstrated that the release of IL-6 and IL-8 is elevated in the adipocytes of individuals with obesity [15]. This may be of significance as either a circulating hormone or as a local regulator of insulin activity. While various studies have investigated the function of TNF-α in insulin resistance, only a limited number have recruited human volunteers. Moreover, no study has ever done a full assessment of cytokine expression, including the evaluation of insulin resistance, in obese patients [16,17].
Conclusion
Sex hormone levels get higher in a weight-dependent manner and in the presence of PCOS situation. The regulation of immunity markers is also enhanced in the same way.
Authors' Contribution
(A) Study Design · (B) Data Collection . (C) Statistical Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search · (G) No Fund Collection
References
- Itriyeva K. The effects of obesity on the menstrual cycle. Curr Probl Pediatr Adolesc Health Care. 2022;52(8):101241.
- Joham AE, Norman RJ, Stener-Victorin E, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668-680.
- Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94(2):106-113.
- Donath MY, Meier DT, Böni-Schnetzler M. Inflammation in the pathophysiology and therapy of cardiometabolic disease. Endocr Rev. 2019;40(4):1080-1091.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87-91.
- Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001.
- Yang J, Chen C. Hormonal changes in PCOS. J Endocrinol. 2024;261(1).
- Abbas AN, Al-Masaoodi RA, Hassan S, et al. The relationship between hormonal levels and hematological parameters in cystic ovarian syndrome. J Med Life. 2023;16(6):937.
- McCartney CR, Campbell RE, Marshall JC, et al. The role of gonadotropin‐releasing hormone neurons in polycystic ovary syndrome. J Neuroendocrinol. 2022;34(5):e13093.
- Han Y, Wu H, Sun S, et al. Effect of high fat diet on disease development of polycystic ovary syndrome and lifestyle intervention strategies. Nutr. 2023;15(9):2230.
- Wang J, Wu D, Guo H, et al. Hyperandrogenemia and insulin resistance: The chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940.
- Hassan SM, al-Jaf AN, Hussien YA, et al. The potential antiviral activity of a novel pyrimidine derivative against Herpes Simplex Virus type-1 (HSV-1). Rev Pharm. 2020;11:795-806.
- Mishima Y, Kuyama A, Tada A, et al. Relationship between serum tumor necrosis factor-α and insulin resistance in obese men with Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2001;52(2):119-123.
- Cardoso NS, Ribeiro VB, Dutra SG, et al. Polycystic ovary syndrome associated with increased adiposity interferes with serum levels of TNF-alpha and IL-6 differently from leptin and adiponectin. Arch Endocrinol Metab. 2020;64:4-10.
- Zhang Y, Lv P, Li Y, et al. Inflammatory Cytokine Interleukin-6 (IL-6) Promotes the Proangiogenic Ability of Adipose Stem Cells from Obese Subjects via the IL-6 Signaling Pathway. Curr Stem Cell Res Ther. 2023;18(1):93-104.
- Hassan SM, Obeid HA, Hasan IS, et al. Etanercept ameliorated cerebral damage during global cerebral ischemia-reperfusion injury in male rats. Azerbaijan Pharmaceut Pharmacoth J. 2023;22(1):53-58.
- Yousif J, Hussein Z, Naser K. The detection of Toxoplasmosis infection among married women in Al-Abbasiya–Najaf region. Al-Kufa Uni J Biol. 2010;2(2).
Author Info
Nadia Kamile Al-Mashta1*, Suzan Hammed Uraibi1, Athraa Imad Habeeb2, Shaima M. Al Sabty1,3 and Saif M. Hassan42Department of Prosthodontic, College of Dentistry, University of Babylon, Babylon, Iraq
3College of Dentistry, Hilla University College, Babylon Governorate, Iraq
4Department of Medical Laboratory Technology, College of Health and Medical Technology, Hilla University College, Babylon, Iraq
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.