Research Article - (2024) Volume 19, Issue 2
3rd generation cephalosporin resistance toward microorganisms found in urine and vaginal discharges in Iraqi women in the mid Euphrates region
Emad Sadiq Ali Alhili1, Rawaa Behlul1, Alia Essa Bashbosh2, Muntadher Zyara3, Nabaa Hussain Hameed Alsaedy3, Ali Saleem Abdulridha Aridhee3 and Saif M. Hassan5*Received: 03-Jun-2024, Manuscript No. gpmp-24-143170; Editor assigned: 04-Jun-2024, Pre QC No. P-143170; Reviewed: 17-Jun-2024, QC No. Q-143170; Revised: 21-Jun-2024, Manuscript No. R-143170; Published: 28-Jun-2024
Abstract
This study intends to examine antimicrobial resistance patterns especially in vaginal swab samples and urine from patients at Al-Sader Hospital as hospitalized patients by standard microbiological technique from April to June of 2023, in order to address the major worldwide threat posed by antimicrobial resistance to the treatment of bacterial infections, particularly in low- and middle-income regions like Iraq. A total number of 200, samples were collected for the detection of pathogenic bacteria; all clinical samples were collected from patient urine, urethral or cervical discharge, pus/swab from wound, ear discharge, nasal and throat swab. From all bacterial isolates 82.89% were found resist to cefixime while (3.28%) recorded intermediate and (13.81%) recorded suspectable, but (70.39%) recorded resistant against ceftriaxone of the all-bacterial isolates and both intermediate and susceptible were (7.89%). The resistance of bacteria is on the rise, because the results showed that most of the isolated bacterial strains are resistant to the third generation of cephalosporins. Accordingly, the recommendations were that it is important to choose the appropriate drugs based on the results of the antibiotic resistance susceptibility test.
Keywords
Vaginal discharge; Bacteria; Cephalosporins
Introduction
Antimicrobial Resistance (AMR) is one of the developing problems in the recent century and is the most severe hazards to worldwide public health [1]. It places a significant problem on everyone, in terms of patient morbidity and economic cost. Increasing AMR pathogens can cause a great percent of hospital acquired infections and medical difficulties around the globe, yet the matter established slight concern by health care segments [2], the number of resistant microbial strains in each organism affected by resistance of the drugs that can escalate the extent of resistance [3]. In low income countries, illogical uses of antimicrobials, drugs availability over the counter and absence of the antimicrobial sensitivity tests in the clinical microbiological laboratories result in high incidence of infections that made the AMR is challenge [4].
In low income countries, illogical uses of antimicrobials, drugs availability over the counter and absence of the antimicrobial sensitivity tests in the clinical microbiological laboratories result in high incidence of infections that made the AMR is challenge [5]. This can adversely affect treatment outcomes, disease spread, and duration of illnesses and costs that affected in serious manner on the future of chemotherapies [6]. The drug companies, clinicians, researchers and public who are seeking of effective drugs are facing difficulties due to bacterial susceptibility to drugs [7], The bacterial resistance crisis has been significantly attributed to the abuse and overuse of antibiotics, The epidemiology of a resistance offers valuable information for preclusion and supports clinicians to prescribe the operative antibiotic therapy, in addition to adjust the use of antibiotics is important in control of drug resistance [8]. For this reason, study was performed to compare between cephalosporins as most appropriate treatment for infections in Najaf city.
Methods
Specimen collection and bacterial identification: A number of 200 samples were collected during the stated time. For the discovery of bacteria that are pathogen, all clinical samples were collected from patient admitted to Al-Sader Hospital as hospitalized patients by typical microbiological technique [9]. The swab/ pus from urine, ear /discharge, wound, nasal or throat swab, urethral or cervical discharge and seminal fluid were used as specimens. According to the samples, sources, each sample were platted onto Mannitol Salt agar, MacConkey agar, Blood agar, Thayer martin agar (Oxoid, UK), after that aerobically incubated for twenty-four hours at thirty seven centigrade. The Species of bacteria were recognized as per the standard microbiological methods [9].
Antimicrobial susceptibility testing: The test of susceptibility was performed on isolates using the agar disc-diffusion technique technique according to Kirby–Bauer method on Mueller–Hinton agar (Oxoid, England) [10]. Accordingly, at least 3 to 5 colonies which well-isolated from the similar morphological kind were carefully chosen from an agar plate culture then transferred and incubated into Muller Hinton broth twenty-four hours at thirty-seven centigrade. The suspension’s turbidity was typically comparable to that of the 0.5 McFarland standards by adjusting with sterile saline. After that, the swab was marked over the whole surface of freshly prepared Mueller Hinton agar plate. The disks that contained the antimicrobial were applied within fifteen minutes to the plates after inoculation and then incubated for twenty-four hours at thirty-seven centigrade. The inhibited region was measured and the sensitive, resistant, or intermediate state were read as results based on
Clinical and Laboratory Standards Institute [11] the antibiotics tested were third-generation cephalosporin: ceftriaxone (30 μg) and cefixime (30 μg).
Data Analysis Descriptive analysis of this study was used frequencies and mean. Data were analysed by Statistical Package for Social Science (SPSS) version 16. The statistically significant differences were considered at P value <0.05 and the results were presented using tables.
Results
A total of 200 clinical specimens were obtained from included urine, sputum, semen, high vaginal swab (Hvs), throat swab and wound swab, from in patient in Al-Sader hospital in AL-Najaf city in Iraq. Among the different clinical samples were tested, a total of 152 (72.38%) were positive bacterial growth. 21.71% Urine, 4.61% Ear swab, 20.39% Hvs, 3.29% Wound, 0.66%, sputum, and 0.66% Throat. Among the bacterial growth, 31.58% E. coli, 15.79% Klebsiella spp, 1.32% Proteus spp, 6.58% Pseudomonas aeruginosa, 1.97% Neisseria gonorrhea, 20.39% Staph aureus, 10.53% Staph saprophytic, and 11.84% Streptococcus spp, Fig. 1. The resistant of bacterial isolates to cefixime was 126 (82.89%), 5 (3.28%) was intermediate and 21(13.81%) was susceptible while 107 (70.39%) was highly resistant against ceftriaxone of the all-bacterial isolates and 12(7.89%) as intermediate and 12(7.89%) was susceptible.
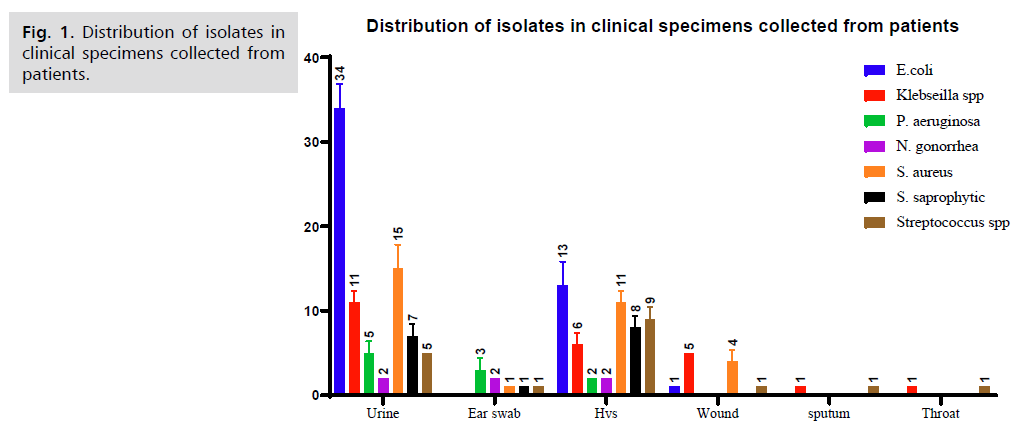
Fig 1. Distribution of isolates in clinical specimens collected from patients.
The bacteria that exhibited the most resistance to antibiotics was E. coli, which showed high resistance to 33 cefixime (73.33%) and 27 ceftriaxone (60.66%), whereas Staph aureus showed strong resistance to 30 cefixime (100%) and 29 ceftriaxone (96.66%) (Tab. 1.).
| Clinical Isolates | Ceftriaxone | Cefixime | ||||
|---|---|---|---|---|---|---|
| Resistant | Intermediate | Susceptible | Resistant | Intermediate | Susceptible | |
| E. coli | 27(60%) | - | 18(40%) | 33(73.33%) | 5(11.11%) | 7(15.55%) |
| Klebsiella | 15(62.5%) | 8(33.33%) | 1(4.1%) | 19(79.16%) | - | 5(20.83%) |
| Proteus | 2(100%) | - | - | 1(50%) | - | 1(50%) |
| pseudomonas | 5(50%) | - | 5(50%) | 6(60%) | - | 4(40%) |
| N. gonorrhoeae | 2(1(66.66%) | 1(33.33%) | 3(100%) | - | - | |
| Staph aureus | 29(96.66%) | - | 1(3.33%) | 30(100%) | - | 0 |
| Staph saprophyt | 20(100%) | 20(100%) | - | - | ||
| Streptococcus spp | 9(50%) | 2(11.11) | 7(38.88) | 14(77.77%) | - | 4(22.22%) |
| Total | 107(70.39%) | 12(7.89%) | 33(7.89%) | 126(82.89%) | 5(3.28%) | 21(13.81%) |
Tab. 1. Pattern of Resistance in the different clinical isolates to antibiotics.
Discussion
In the twenty-first century, the problem of antibiotic resistance is a steadily increasing problem and represents the greatest threat to public health on a global basis. Hence, disease agents once believed to exist to be vulnerable to antibiotics come back in new leagues Resistance to these treatments [12].
This study showed that the isolates taken from female were resistant for third-generation cephalosporin was not significant in isolates taken from female, where the resistance to third-generation antibiotics was similar to the patron for both types of treatment under study. This result is identical to the results of previous research [13].
The antibiotic resistance pattern differs for the same type of bacterial isolates based on the type of the sample. According to this study, no significant difference was observed for all bacterial isolates in their resistance to both types of antibiotics under study (ceftriaxone, cefixime). The majority of these E. coli isolates were isolated from urinary tract infections and were mostly elevated for both types of antigens (Fig. 1.). This type of bacteria has a wide history of antibiotic resistance, causing difficulty in treating vaginal and urinary tract infections [12], which makes it a strong candidate for further development of its resistance to treatment.
In addition, the E. coli isolated from the rest of the samples showed clear resistance to the third-generation cephalosporin. This is what was mentioned in many previous researches [14,15], in the research that was carried out in some researches it was shown that the resistance of E. coli to ceftriaxone was at higher rates than its resistance to cefixime [16,17].
Most of the staphylococci in this study were resistant to both third-generation antibiotics without a significant difference for both types of antibiotics, and this result does not match a number of other studies that showed that staphylococcal strains are resistant to ceftriaxone at higher rates than their resistance to cefixime. And the search showed [18]. The research under study showed that Staphylococcus aureus, in the majority of its number, was isolated from urinary tract infections and vaginal infections. Causing urinary and vaginal infections [19], and this indicates with certainty that this type of isolate continues to develop resistance to antibiotics, especially antibiotics of the third generation of cephalosporins [20].
The results of this study are S. aureus isolates that have the same ability to resist the antibiotics under study compared to S. aureus, and this is not similar to the results of previous research, which showed that S. aureus has an ability to resist treatment that exceeds the ability of other Staphylococcus types [21].
Most of Klebsiella isolates in this research are resistant to both types of third-generation antibiotics, but they show higher resistance to cefixime compared to ceftriaxone, and this result was not identical to other previous research that claimed that they are resistant to third-generation antibiotics in the same way [22].
As for isolates of Pseudomonas aeruginosa, Proteus bacteria, and Streptococcus aureus, they are resistant to ceftriaxone at a higher rate than cefixime. This result agreed with other research results [16,23].
Conclusion
Significant increase in bacterial resistance to the third generation cephalosporins and these same third-generation resistant strains are based on many other types of antibiotics, which makes it difficult to determine effective treatment. This study recommends interest in collecting data of antibiotic-resistant bacterial isolates and using them in choosing the right treatment.
Funding
This study did not receive any funding.
Ethical Clearance
The protocol of a study has been accepted as stated by the Ethical Committee in the Najaf Health Directorate on 2-10-2019. In addition, before taking the sample; verbal agreement was gotten from the patients. Health safety was engaged during sampling. This study was done according to the Iraqi Ministry of Health Ethics Committee also it was agreed with all national regulations.
Authors’ Contribution
Designed the study and drafted the manuscript: HDSS. Collected data and performed background literature review: HDSS. Performed statistical analysis: SKN. Supervised results and discussion: EMK, IH and GH. All authors reviewed and approved the final version of the manuscript.
Acknowledgment
The authors would like to thank all the supervisors and all the research participants who willingly attended on the data collection, enabling the successful implementation of this study.
Conflict of Interest
The authors declared there is no conflict of interest.
References
- Roope LS, Smith RD, Pouwels KB, et al. The challenge of antimicrobial resistance: what economics can contribute. Science. 2019;364(6435):eaau4679.
- AL-Khikani FH. Antimicrobial resistance profile among major bacterial pathogens in Southern Babil, Iraq. Galician Med J. 2020;27(3):E202036-.
- Spruijt P, Petersen AC. Multilevel governance of antimicrobial resistance risks: A literature review. J Risk Res. 2022;25(8):945-958.
- AL-Khikani FH, Kadim BJ, Ayit AS, et al. Evaluation cephalosporins resistance in pathogenic bacteria isolated clinically. World News Nat. Sciences. 2020;31.
- Govender R, Amoah ID, Adegoke AA, et al. Identification, antibiotic resistance, and virulence profiling of Aeromonas and Pseudomonas species from wastewater and surface water. Environ Monit Assess. 2021;193(5):294.
- Haider A, Ikram M, Rafiq A. Antimicrobials; drug resistance. InGreen nanomaterials as potential antimicrobials Cham: Springer International Publishing. 2022: 109-124.
- Serwecińska L. Antimicrobials and antibiotic-resistant bacteria: a risk to the environment and to public health. Water. 2020;12(12):3313.
- Hasan CM, Dutta D, Nguyen AN. Revisiting antibiotic resistance: mechanistic foundations to evolutionary outlook. Antibiotics. 2021;11(1):40.
- Abbey TC, Deak E. What's new from the CLSI subcommittee on antimicrobial susceptibility testing M100. Clin Microbiol Newsl. 2019;41(23):203-209.
- Drew WL, Barry AL, O'Toole R, et al. Reliability of the Kirby-Bauer disc diffusion method for detecting methicillin-resistant strains of Staphylococcus aureus. Appl Microbiol.1972;24(2):240-247.
- Blazevic DJ, Koepcke MH, Matsen JM. Quality Control Testing with the Disk Antibiotic Susceptibility Test of Bauer–Kirby–Sherris–Turck. Am J Clin Pathol. 1972;57(5):592-597.
- Watson JR, Burch C, Leber AL. Surrogate testing of oral third-generation cephalosporin susceptibility to common uropathogens. Diagn Microbiol Infect Dis. 2021;99(4):115299.
- Ajmal M, Zamir A, Rehman AU, et al. Clinical pharmacokinetics of cefixime: A systematic review. Xenobiotica. 2023;53(3):149-162.
- Al-Tamimi M, Albalawi H, Shalabi M, et al. Cefixime and cefixime-clavulanate for screening and confirmation of extended-spectrum beta-lactamases in Escherichia coli. Ann Clin Microbiol Antimicrob. 2022;21(1):20.
- Li X, Jia P, Zhu Y, et al. Establishment of epidemiological cut-off values for cefoselis, a new fourth-generation cephalosporin, against Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Proteus mirabilis and Pseudomonas aeruginosa. J Antimicrob Chemother. 2021;76(10):2593-2599.
- Sokhn ES, Salami A, El Roz A, et al. Antimicrobial susceptibilities and laboratory profiles of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates as agents of urinary tract infection in Lebanon: paving the way for better diagnostics. Med Sci. 2020;8(3):32.
- Thelen H, Dilworth TJ, Mercier RC. Examining the Combination of Cefixime and Amoxicillin/Clavulanate against Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Isolates. Chemotherapy. 2022;67(4):261-268.
- Wangoye K, Mwesigye J, Tungotyo M, et al. Chronic wound isolates and their minimum inhibitory concentrations against third generation cephalosporins at a tertiary hospital in Uganda. Sci Rep. 2022;12(1):1195.
- Middlebusher LR. Antibotic Resistance in Africa: Escherichia coli and Staphylococcus aureus. Microrev Mol Cell Biol. 2023;1.
- Ali EA, Alshuaibi ON, Alsweedi KS. Staphylococcus aureus resistance against cephalosporin antibiotic in aden-yemen. EJUA-BA. 2021;2(3):139-144.
- van Prehn J, van Triest MI, Altorf-van Der Kuil W, et al. Third-generation cephalosporin and carbapenem resistance in Streptococcus mitis/oralis. Results from a nationwide registry in the Netherlands. Clin Microbiol Infect. 2019;25(4):518-520.
- Shalaan AH, Muhammed AJ, Mohammed RM, et al. The effects of cephalosporin antibiotics generations on samples taken from different cases (Oral cavity and surgical wounds).
- Viala B, Zaidi FZ, Bastide M, et al. Assessment of the in vitro activities of ceftolozane/tazobactam and ceftazidime/avibactam in a collection of beta-lactam-resistant Enterobacteriaceae and Pseudomonas aeruginosa clinical isolates at Montpellier University Hospital, France. Microb Drug Resist. 2019;25(9):1325-1329.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Author Info
Emad Sadiq Ali Alhili1, Rawaa Behlul1, Alia Essa Bashbosh2, Muntadher Zyara3, Nabaa Hussain Hameed Alsaedy3, Ali Saleem Abdulridha Aridhee3 and Saif M. Hassan5*2Department of Medical Microbiology, Faculty of Medicine, University of Kufa, Kufa, Iraq
3Department of Clinical Laboratory Sciences, Faculty of Pharmacy, University of Kufa, Kufa, Iraq
4College of Pharmacy, The Islamic University, Najaf 54001, Iraq
5Department of Pharmacy, Al-Zahrawi University College, Karbala, Iraq
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.